Label: TRETINOIN capsule
- NDC Code(s): 10370-268-01
- Packager: Endo USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TRETINOIN CAPSULES safely and effectively. See full prescribing information for TRETINOIN CAPSULES.
TRETINOIN capsules, for oral use
Initial U.S. Approval: 1995WARNING: EMBRYO-FETAL TOXICITY and DIFFERENTIATION SYNDROME
See full prescribing information for complete boxed warning.
- Embryo-Fetal Toxicity: tretinoin capsules can cause embryo-fetal loss and malformations when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Females of reproductive potential must have a negative pregnancy test before initiating tretinoin capsules. Advise females of reproductive potential to use two effective methods of contraception during treatment with tretinoin capsules and for 1 month after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with tretinoin capsules and for 1 week after the last dose. (5.1, 8.1, 8.3)
- Differentiation Syndrome, which can be life-threatening or fatal, occurred in about 26% of patients with APL who received tretinoin capsules. At first signs or symptoms of this syndrome, immediately initiate high-dose corticosteroid therapy and hemodynamic monitoring until resolution of signs and symptoms. Consider withholding tretinoin capsules for moderate and severe Differentiation Syndrome until resolution. (5.2)
INDICATIONS AND USAGE
Tretinoin capsules are a retinoid indicated for induction of remission in adults and pediatric patients 1 year of age and older with acute promyelocytic leukemia (APL), characterized by presence of t(15;17) translocation or presence of PML/RARα gene expression, and who are refractory to or who have relapsed from anthracycline chemotherapy or for whom anthracycline-based chemotherapy is contraindicated. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Capsules: 10 mg (3)
CONTRAINDICATIONS
Hypersensitivity to tretinoin capsules, any of its components, or other retinoids (4)
WARNINGS AND PRECAUTIONS
- Patients Without t(15;17) Translocation or PML/RARα Fusion: tretinoin capsules may be initiated based on morphological diagnosis of APL. Confirm diagnosis by detection of the t(15;17) translocation or PML/RARα fusion. (5.3)
- Leukocytosis: Consider administering cytoreductive chemotherapy (including an anthracycline if not contraindicated or hydroxyurea) with tretinoin capsules in the setting of leukocytosis, as clinically indicated. (5.4)
- Intracranial Hypertension: Tretinoin capsules has been associated with benign intracranial hypertension, especially in pediatric patients. Consider interruption, dose reduction, or discontinuation of tretinoin capsules as appropriate. (5.5)
- Lipid Abnormalities: Patients experienced hypercholesterolemia and/or hypertriglyceridemia, which may be reversible upon completion of treatment. Monitor fasting triglycerides and cholesterol at baseline and periodically during treatment. (5.6)
- Hepatotoxicity: Monitor liver function test results at baseline and during treatment as clinically indicated. (5.7)
- Thromboembolic Events: Venous and arterial events have been reported; these events may occur during the first month of treatment with tretinoin capsules. (5.8, 7.4)
ADVERSE REACTIONS
The most common adverse reactions (≥30%) are headache, fever, skin/mucous membrane dryness, bone pain, malaise, shivering, upper respiratory tract disorders, dyspnea, hemorrhage, infections, nausea/vomiting, rash, peripheral edema, leukocytosis, pain, gastrointestinal hemorrhage, chest discomfort, abdominal pain (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Endo at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Strong CYP3A Inhibitors and Inducers: Avoid coadministration with strong CYP3A inhibitors and inducers. (7.1)
- Concomitant Use of Products Known to Cause Intracranial Hypertension: Avoid concomitant use with other products that can cause intracranial hypertension. (7.2)
- Vitamin A: Avoid concomitant use with vitamin A. (7.3)
- Anti-fibrinolytic Agents: Avoid concomitant use with anti-fibrinolytic agents. (7.4)
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Safety Information
2.2 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
5.2 Differentiation Syndrome
5.3 Patients Without t(15;17) Translocation or PML/RARα Fusion
5.4 Leukocytosis
5.5 Intracranial Hypertension
5.6 Lipid Abnormalities
5.7 Hepatotoxicity
5.8 Thromboembolic Events
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Tretinoin Capsules
7.2 Concomitant Use of Products Known to Cause Intracranial Hypertension
7.3 Vitamin A
7.4 Anti-fibrinolytic Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Use in Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY and DIFFERENTIATION SYNDROME
- Tretinoin capsules can cause embryo-fetal loss and malformations when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Females of reproductive potential must have a negative pregnancy test before initiating tretinoin capsules. Advise females of reproductive potential to use two effective methods of contraception during treatment with tretinoin capsules and for 1 month after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with tretinoin capsules and for 1 week after the last dose [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
- Differentiation Syndrome, which can be life-threatening or fatal, occurred in about 26% of patients with APL who received tretinoin capsules. At first signs or symptoms of this syndrome, immediately initiate high-dose corticosteroid therapy and hemodynamic monitoring until resolution of signs and symptoms. Consider withholding tretinoin capsules for moderate and severe Differentiation Syndrome until resolution [see Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE
Tretinoin capsules are indicated for the induction of remission in adults and pediatric patients 1 year of age and older with acute promyelocytic leukemia (APL) characterized by the presence of the t(15;17) translocation or PML/RARα gene expression, and who are refractory to or who have relapsed from anthracycline chemotherapy or for whom anthracycline-based chemotherapy is contraindicated.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Safety Information
Verify pregnancy status in females of reproductive potential prior to initiating tretinoin capsules. Females of reproductive potential must have a negative pregnancy test before initiating tretinoin capsules [see Use in Specific Populations (8.3)].
2.2 Recommended Dosage
The recommended dosage of tretinoin capsules is 22.5 mg/m2 orally twice daily until complete remission is documented. Discontinue tretinoin capsules 30 days after achievement of complete remission or after 90 days of treatment, whichever occurs first.
Discontinue tretinoin capsules if the t(15;17) translocation or PML/RARα fusion has not been identified [see Warnings and Precautions (5.3)].
Take tretinoin capsules with a meal.
Swallow tretinoin capsules whole with water. Do not chew, dissolve, or open capsule.
Do not take a missed dose of tretinoin capsules unless it is more than 10 hours until the next scheduled dose.
If vomiting occurs after tretinoin capsules administration, do not take an additional dose, but continue with the next scheduled dose.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Tretinoin capsules are contraindicated in patients with a known hypersensitivity to tretinoin capsules, any of its components, or other retinoids. Reactions have included rash, pruritus, face edema, and dyspnea. [see Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
Tretinoin capsules can cause embryo-fetal loss and malformations when administered to a pregnant woman. Tretinoin capsules are a retinoid and there is an increased risk of major congenital malformations, spontaneous abortions and premature births following exposure to retinoids during pregnancy in humans. Tretinoin has teratogenic and embryotoxic effects in mice, rats, hamsters, rabbits and pigtail monkeys at doses less than the human dose on a mg/m2 basis.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use 2 effective methods of contraception during treatment with tretinoin capsules and for 1 month following the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with tretinoin capsules and for 1 week following the last dose [see Use in Specific Populations (8.1, 8.3)].
5.2 Differentiation Syndrome
Differentiation Syndrome, which may be life-threatening or fatal, occurred in about 26% of patients with APL who received tretinoin capsules [see Adverse Reactions (6.1)]. Symptoms include fever, dyspnea, acute respiratory distress, weight gain, radiographic pulmonary infiltrates, pleural and pericardial effusions, edema, and hepatic, renal, and multi-organ failure. This syndrome has been accompanied by impaired myocardial contractility and episodic hypotension and it has been observed with or without concomitant leukocytosis. This syndrome generally occurs during the first month of treatment, as early as following the first dose. Endotracheal intubation and mechanical ventilation were required in some cases due to progressive hypoxemia and several patients have died with multi-organ failure.
At the first signs or symptoms of this syndrome, immediately administer dexamethasone 10 mg intravenously every 12 hours until signs and symptoms have abated for at least 3 days and initiate hemodynamic monitoring until resolution of signs and symptoms. Consider withholding tretinoin capsules for moderate and severe differentiation syndrome until resolution [see Adverse Reactions (6.1)].
5.3 Patients Without t(15;17) Translocation or PML/RARα Fusion
Tretinoin capsules may be initiated based on the morphological diagnosis of acute promyelocytic leukemia (APL). Confirm the diagnosis of APL by detection of the t(15;17) translocation using cytogenetic studies or PML/RARα fusion using molecular diagnostic techniques. Tretinoin capsules not recommended for use in patients without these genetic markers [see Indications and Usage (1)].
5.4 Leukocytosis
Rapidly evolving leukocytosis, which can be life-threatening, occurred in about 40% of patients with APL who received tretinoin capsules [see Adverse Reactions (6.1)]. Patients who present with a baseline white blood cell count (WBC) > 5 × 109/L have an increased risk. Patients who receive chemotherapy with tretinoin capsules may be at a reduced risk. Rapidly evolving leukocytosis is associated with a higher risk of life-threatening complications.
Consider administering cytoreductive chemotherapy (including an anthracycline if not contraindicated or hydroxyurea) with tretinoin capsules in the setting of leukocytosis, as clinically indicated.
5.5 Intracranial Hypertension
Retinoids, including tretinoin capsules, have been associated with intracranial hypertension, especially in pediatric patients. Early signs and symptoms include papilledema, headache, nausea, vomiting, and visual disturbances. Evaluate patients with these symptoms for intracranial hypertension, and, if present, institute appropriate care in concert with neurological assessment. Consider interruption, dose reduction, or discontinuation of tretinoin capsules as appropriate.
The concomitant use of other products (e.g., tetracyclines) that can cause intracranial hypertension may increase the risk. Avoid concomitant use of tretinoin capsules with other products that can cause intracranial hypertension [see Drug Interactions (7.2)].
5.6 Lipid Abnormalities
Hypercholesterolemia and/or hypertriglyceridemia has occurred in up to 60% of patients who received tretinoin capsules. These changes may be reversible upon completion of treatment. The clinical consequences of increased triglycerides and cholesterol are unknown, but venous thrombosis and myocardial infarction have been reported in patients who ordinarily are at low risk for such complications.
Monitor fasting triglycerides and cholesterol at baseline and periodically during treatment.
5.7 Hepatotoxicity
Elevated liver function test results occurred in 50% to 60% of patients during treatment with tretinoin capsules. Most of these abnormalities resolved without interruption of tretinoin capsules or after completion of treatment.
Monitor liver function test at baseline and during treatment as clinically indicated. Consider withholding tretinoin capsules if liver function test results increase to greater than 5 times the upper limit of normal values until resolution.
5.8 Thromboembolic Events
Venous and arterial thromboembolic events, including cerebrovascular accident, myocardial infarction and renal infarct have been reported with tretinoin capsules [see Adverse Reactions (6.2)]. These events may occur during the first month of treatment. Patients taking anti-fibrinolytic agents may have an increased risk.
Avoid concomitant use of tretinoin capsules and anti-fibrinolytic agents, such as tranexamic acid, aminocaproic acid or aprotinin [see Drug Interactions (7.4)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Differentiation Syndrome [see Warnings and Precautions (5.2)]
- Leukocytosis [see Warnings and Precautions (5.4)]
- Intracranial hypertension [see Warnings and Precautions (5.5)]
- Lipid abnormalities [see Warnings and Precautions (5.6)]
- Hepatotoxicity [see Warnings and Precautions (5.7)]
- Thromboembolic events [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Acute Promyelocytic Leukemia
The safety of tretinoin capsules was evaluated in patients with APL who received tretinoin capsules at a dose of 22.5 mg/m2 orally twice daily [see Clinical Studies (14)].
The most common adverse reactions (≥30%) were headache, fever, skin/mucous membrane dryness, bone pain, malaise, shivering, upper respiratory tract disorders, dyspnea, hemorrhage, infections, nausea/vomiting, rash, peripheral edema, leukocytosis, pain, gastrointestinal hemorrhage, chest discomfort, abdominal pain.
Table 1 summarizes the adverse reactions for patients with APL.
Table 1. Adverse Reactions (≥ 10%) Occurring in Patients with APL Who Received Tretinoin Capsules
Adverse Reaction Tretinoin Capsules All Grades
(%)Nervous system disorders Headache 86 Dizziness 20 Paresthesias 17 Anxiety 17 Insomnia 14 Depression 14 Confusion 11 General disorders Fever 83 Skin/mucous membrane dryness 77 Malaise 66 Shivering 63 Peripheral edema 52 Pain 37 Chest discomfort 32 Edema 29 Mucositis 26 Weight increase 23 Anorexia 17 Weight decrease 17 Musculoskeletal and connective tissue disorders Bone pain 77 Myalgia 14 Respiratory, thoracic and mediastinal disorders Upper respiratory tract disorders 63 Dyspnea 60 Respiratory insufficiency 26 Pleural effusion 20 Rales 14 Expiratory wheezing 14 Pneumonia 14 Vascular disorders Hemorrhage 60 Gastrointestinal hemorrhage 34 Flushing 23 Hypotension 14 Hypertension 11 Phlebitis 11 Infections and infestations Infections 58 Gastrointestinal disorders Nausea/vomiting 57 Abdominal pain 31 Other gastrointestinal disorders 26 Diarrhea 23 Constipation 17 Dyspepsia 14 Abdominal distention 11 Skin and subcutaneous tissue disorders Rash 54 Pruritus 20 Increased sweating 20 Alopecia 14 Skin changes 14 Blood and lymphatic system disorders Leukocytosis 49 Differentiation syndrome1 26 Disseminated intravascular coagulation 26 Ear and labyrinth disorders Earache or feeling of fullness in the ears 23 Cardiac disorders Arrhythmias 23 Eye disorders Visual disturbances 17 Ocular disorders 17 Renal and urinary disorders Renal insufficiency 11 1Differentiation syndrome can be associated with other commonly reported events
such as fever, leukocytosis, dyspnea, pneumonia, pleural effusion, pericardial effusion,
hypotension, edema, weight gain, and renal failure.Adverse reactions that occurred in <10% of patients who received tretinoin capsules include:
- Hepatobiliary disorders: Hepatosplenomegaly (9%), hepatitis (3%), unspecified liver disorder (3%).
- Musculoskeletal and connective tissue disorders: Flank pain (9%), bone inflammation (3%).
- Nervous system disorders: Agitation (9%), cerebral hemorrhage (9%), intracranial hypertension (9%), hallucination (6%), abnormal gait, agnosia, aphasia, asterixis, cerebellar edema, cerebellar disorders, convulsions, coma, CNS depression, dysarthria, encephalopathy, facial paralysis, hemiplegia, hyporeflexia, hypotaxia, no light reflex, neurologic reaction, spinal cord disorder, stroke, tremor, leg weakness, unconsciousness, dementia, forgetfulness, somnolence, and slow speech (3% each).
- Renal and urinary disorders: Dysuria (9%), acute renal failure, micturition frequency, renal tubular necrosis, and enlarged prostate (3% each).
- Respiratory, thoracic and mediastinal disorders: Lower respiratory tract disorders (9%), pulmonary infiltration (6%), bronchial asthma, pulmonary edema, larynx edema, and unspecified pulmonary disease (3% each).
- Infections and infestations: Cellulitis (8%).
- Blood and lymphatic system disorders: Lymph disorders (6%).
- Cardiovascular disorders: Cardiac failure (6%), cardiac arrest, myocardial infarction, enlarged heart, heart murmur, myocarditis, pericarditis, and secondary cardiomyopathy (3% each).
- Ear and labyrinth disorders: Hearing loss and other unspecified auricular disorders (6%), irreversible hearing loss (<1%).
- General disorders: Face edema (6%), pallor (6%), hypothermia (3%).
- Metabolism and nutrition disorders: Fluid imbalance (6%), acidosis (3%).
- Eye disorders: Changed visual acuity (6%), visual field defects (3%).
- Gastrointestinal disorders: Ascites, ulcer (3% each).
- Vascular disorders: Ischemia and pulmonary hypertension (3% each).
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Erythema nodosum, basophilia and hyperhistaminemia, Sweet’s Syndrome, organomegaly, hypercalcemia, pancreatitis, myositis, thrombosis (both venous and arterial), thrombocytosis, genital ulceration and vasculitis, predominantly involving the skin.
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Tretinoin Capsules
Strong CYP3A Inhibitors
The coadministration of tretinoin capsules with ketoconazole, a strong CYP3A4 inhibitor, increased tretinoin plasma concentration, which may increase the risk of adverse reactions [see Clinical Pharmacology (12.3)]. Avoid coadministration of tretinoin capsules with strong CYP3A inhibitors if possible. Monitor patients taking a strong CYP3A inhibitor with tretinoin capsules more frequently for adverse reactions.
Strong CYP3A Inducers
The coadministration of tretinoin capsules with strong CYP3A4 inducers may decrease tretinoin plasma concentrations, which may reduce its efficacy. Avoid coadministration with strong CYP3A inducers if possible.
7.2 Concomitant Use of Products Known to Cause Intracranial Hypertension
Intracranial hypertension has been reported in patients who received tretinoin capsules and concomitant use of other products that can cause intracranial hypertension, such as tetracyclines, may increase the risk. Avoid concomitant use of tretinoin capsules with other products agents that can cause intracranial hypertension [see Warnings and Precautions (5.5)].
7.3 Vitamin A
The concomitant use of vitamin A with tretinoin capsules may lead to vitamin A related adverse reactions. Avoid concomitant use of tretinoin capsules with vitamin A.
7.4 Anti-fibrinolytic Agents
Fatal thrombotic complications have been reported in patients who have received tretinoin capsules and concomitant use of anti-fibrinolytic agents may increase the risk. Avoid concomitant use of tretinoin capsules with anti-fibrinolytic agents [see Warnings and Precautions (5.8)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings in animals and its mechanism of action [see Clinical Pharmacology (12.1)], tretinoin capsules can cause embryo-fetal loss and malformations when administered to a pregnant woman. Tretinoin capsules are a retinoid and there is an increased risk of major congenital malformations, spontaneous abortions and premature births following exposure to retinoids during pregnancy in humans. Tretinoin was teratogenic and embryotoxic in mice, rats, hamsters, rabbits and pigtail monkeys at doses less than the human dose on a mg/m2 basis (see Data). Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the
U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
Trentoin capsules are a retinoid and increased spontaneous abortions and major fetal abnormalities related to the use of retinoids have been documented in humans. Reported malformations include abnormalities of the central nervous system, musculoskeletal system, external ear, eye, thymus and great vessels; and facial dysmorphia, cleft palate, and parathyroid hormone deficiency. Some of these abnormalities were fatal.
IQ scores less than 85, with or without obvious CNS abnormalities, have been reported in pediatrics exposed to retinoids in utero.
Animal Data
Tretinoin causes fetal resorptions and a decrease in live fetuses in all animals studied. Gross external, soft tissue and skeletal alterations occurred at doses higher than 0.7 mg/kg/day in mice, 2 mg/kg/day in rats, 7 mg/kg/day in hamsters, and at a dose of 10 mg/kg/day, the only dose tested, in pigtail monkeys (about 1/20, 1/4, and 1/2 and 4 times the human dose, respectively, on a mg/m2 basis).
8.2 Lactation
There are no data on the presence of tretinoin in human milk, the effects on the breastfeed child or the effects on milk production. Because of the potential for serious adverse reactions from tretinoin capsules in breastfed infants, advise women not to breastfed during treatment with tretinoin capsules and for 1 week after the last dose.
8.3 Use in Females and Males of Reproductive Potential
Tretinoin capsules can cause embryo-fetal loss and malformations when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating tretinoin capsules. Females of reproductive potential must have a negative pregnancy test within 1 week prior to initiating tretinoin capsules with a sensitivity of at least 50 mIU/mL.
Contraception
Females
Advise females of reproductive potential to abstain continuously from sexual intercourse or to use two effective methods of contraception. Counsel patients to use two effective methods of contraception during treatment with tretinoin capsules and for 1 month after the last dose. Two methods of effective contraception is indicated even where there has been a history of infertility, unless due to hysterectomy. Refer females of reproductive potential to a qualified provider of contraceptive methods, if needed.
Males
Advise males with female partners of reproductive potential to use effective contraception during and after treatment with tretinoin capsules and for 1 week after the last dose.
Infertility
Males
Based on testicular toxicities observed in dogs, tretinoin capsules may impair male fertility [see Nonclinical Toxicology (13.1)]. The reversibility of effect on fertility is unknown.
8.4 Pediatric Use
Safety and effectiveness of tretinoin capsules have been established in pediatric patients 1 year of age and older and the information on this use is discussed throughout the labeling. The maximum tolerated dose is lower in pediatric patients compared to adults. Some pediatric patients experience severe headache and intracranial hypertension, which required management with an analgesic and a lumbar puncture. Dose reduction may be considered for pediatric patients experiencing serious and/or intolerable adverse reactions.
Safety and effectiveness in pediatric patients less than 1 year of age have not been established.
-
10 OVERDOSAGE
In case of overdose with tretinoin capsules, reversible signs of hypervitaminosis A (headache, nausea, vomiting, mucocutaneous symptoms) can appear. Overdosage with other retinoids has been associated with transient headache, facial flushing, cheilosis, abdominal pain, dizziness and ataxia. These symptoms have quickly resolved without apparent residual effects.
There is no specific treatment in the case of an overdose; however, it is important that the patient be treated in a special hematological unit.
-
11 DESCRIPTION
Tretinoin is a retinoid. The chemical name is all-trans retinoic acid. The molecular formula is C20H28O2 and the molecular weight is 300.44 g/mol. The structural formula is:
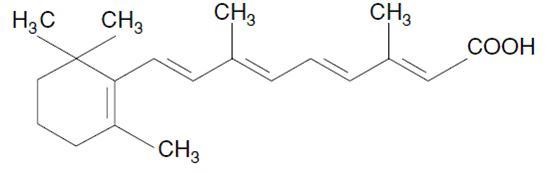
Tretinoin Structural Formula
It is a yellow to light orange, crystalline powder with melting point at about 182°C (with decomposition). Tretinoin is practically insoluble in water, sparingly soluble in methylene chloride, and slightly soluble in ethanol (96%).
Tretinoin capsules are available as capsules containing 10 mg tretinoin for oral use. Each capsule also contains butylated hydroxyanisole, edetate disodium, soybean oil, hydrogenated vegetable oils, medium chain triglycerides, soya lecithin, and yellow beeswax. The gelatin capsule shell contains gelatin, glycerin, yellow iron oxide, red iron oxide and titanium dioxide. Capsules are printed with edible black ink, which consist of propylene glycol, iron oxide black, polyvinyl acetate phthalate and polyethylene glycol 400.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tretinoin induces cytodifferentiation and decreased proliferation of APL cells in culture and in vivo. In APL patients, tretinoin treatment produces an initial maturation of the primitive promyelocytes derived from the leukemic clone, followed by a repopulation of the bone marrow and peripheral blood by normal, polyclonal hematopoietic cells in patients achieving complete remission (CR). The exact mechanism of action of tretinoin in APL is unknown.
12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of tretinoin capsules have not been characterized.
12.3 Pharmacokinetics
Following the administration of tretinoin capsules 22.5 mg/m2 orally twice daily, the mean ± SD peak tretinoin concentrations after the first dose was 394 ± 89 and after 1 week of continuous treatment was 138 ± 139 ng/mL, while area under the curve (AUC) after the first dose was 537 ± 191 ng·h/mL and after 1 week of continuous treatment was 249 ± 185 ng·h/mL.
Absorption
Time to reach peak concentration was between 1 and 2 hours. The absolute bioavailability of tretinoin capsules was approximately 50%.
Effect of Food
The effect of food on the absorption of tretinoin capsules has not been characterized. Food increases the absorption of retinoids, as a class.
Distribution
The apparent volume of distribution of tretinoin has not been determined.
Protein binding is greater than 95%, predominately to albumin. Plasma protein binding remains constant over the concentration range of 10 to 500 ng/mL.
Elimination
The terminal elimination half-life of tretinoin following initial dosing is 0.5 to 2 hours in patients with APL.
Metabolism
Tretinoin induced its own metabolism with plasma concentrations after 1 week of continuous therapy decreased to one-third of their day 1 values. Tretinoin is metabolized by cytochrome P450 enzymes, CYP3A4, 2C8, and 2E and undergoes glucuronidation by UGT2B7. Metabolites include 9-cis retinoic acid, 13-cis retinoic acid, 4-oxo trans retinoic acid, 4-oxo cis retinoic acid, and 4-oxo trans retinoic acid glucuronide. The metabolites 4-oxo retinoic acid and 4-oxo trans retinoic acid glucuronide have one-third of the pharmacological activity of the parent compound.
Excretion
Following administration of radiolabeled tretinoin 2.75 mg and 50 mg (0.53 to 9.6 times the approved recommended dosage based on 1.7 m2), approximately 63% of radioactivity was recovered in the urine within 72 hours and 31% appeared in the feces within 6 days.
Specific Populations
The effect of age, sex, race, renal impairment, and hepatic impairment on the pharmacokinetics of tretinoin is unknown.
Drug Interaction Studies
Clinical Studies
Coadministration of ketoconazole (strong CYP3A inhibitor) increased tretinoin AUC by 72%.
In Vitro Studies
Effect of Tretinoin on Transporters: Tretinoin does not inhibit P-gp and BCRP in vitro.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No 2-year carcinogenicity studies in rodents have been conducted with tretinoin. In a carcinogenicity study, female B5D2F1 mice pretreated with a carcinogen diethylnitrosamine (DEN, intraperitoneal 50 mg/kg and 100 mg/kg) received dietary supplement of all-trans-retinoic acid (tretinoin) for 12 months. Tretinoin at a dose of 30 mg/kg/day in the diet (about 2 times the human dose on a mg/m2 basis) was shown to increase the rate of DEN-induced mouse hepatocellular carcinomas. Tretinoin in combination with 50 mg/kg of DEN also increased the incidence of hemangiomas and hemangiosarcomas.
Tretinoin was negative when tested in the Ames and Chinese hamster V79 cell HGPRT assays for mutagenicity. A 2-fold increase in the sister chromatid exchange (SCE) has been demonstrated in human diploid fibroblasts, but other chromosome aberration assays, including an in vitro assay in human peripheral lymphocytes and an in vivo mouse micronucleus assay, did not show a clastogenic or aneuploidogenic effect.
Adverse effects on fertility and reproductive performance were not observed in studies conducted in rats at doses up to 5 mg/kg/day (about 2/3 the human dose on a mg/m2 basis). In a 6-week toxicology study in dogs, testicular degeneration, with increased numbers of immature spermatozoa, were observed at 10 mg/kg/day (about 4 times the equivalent human dose in mg/m2).
-
14 CLINICAL STUDIES
The efficacy of tretinoin capsules have been evaluated in 114 previously treated patients and in 67 previously untreated (“de novo”) patients with APL in one open-label, uncontrolled single investigator clinical study (Memorial Sloan-Kettering Cancer Center [MSKCC]) and in two cohorts of compassionate cases treated by multiple investigators under the auspices of the National Cancer Institute (NCI). Patients received tretinoin capsules 22.5 mg/m2 orally twice daily for up to 90 days following the first dose or 30 days following achievement of complete remission. Efficacy results are shown Table 2.
Table 2. Efficacy Results in a Controlled Clinical Trial (MSKCC) and Compassionate Use
MSKCC
NCI Cohort 1 NCI Cohort 2 Relapsed
N = 20
De Novo
n = 15
Relapsed*
N = 48
De Novo
n = 14
Relapsed
n = 46
De Novo †
n = 38
Complete
Remission16 (80%) 11 (73%) 24 (50%) 5 (36%) 24 (52%) 26 (68%) Median
Survival(months)
10.8 NR 5.8 0.5 8.8 NR Median
Follow-up(months)
9.9 42.9 5.6 1.2 8.0 13.1 NR = Not Reached
NA = Not Available
*Including 9 chemorefractory patients
†Including 8 patients who received chemotherapy but failed to enter remission
The median time to complete remission was between 40 and 50 days (range: 2 to 120 days). Most patients received cytotoxic chemotherapy during the remission phase.
Ten of 15 pediatric cases achieved complete remission (8 of 10 males and 2 of 5 females).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tretinoin capsules are supplied as 10 mg capsules, two-tone (lengthwise) with reddish-brown opaque and yellow gelatin shell, imprinted with “TR” with black ink on the yellow side. Supplied in high-density polyethylene, opaque bottles of 100 capsules available in:
- 100 count bottles with child-resistant closure NDC 10370-268-01
Store at 20ºC to 25ºC (68ºF to 77ºF); [see USP Controlled Room Temperature].
Keep bottle tightly closed. Protect from light.
-
17 PATIENT COUNSELING INFORMATION
Embryo-Fetal Toxicity
Advise female patients of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].
Advise females of reproductive potential to use 2 methods of effective contraception during treatment with tretinoin capsules and for 1 month after the last dose [see Use in Specific Populations (8.3)].
Advise males with female partners of reproductive potential to use effective contraception during treatment with tretinoin capsules and for 1 week after the last dose [see Use in Specific Populations (8.3)].
Differentiation Syndrome
Advise patients that tretinoin capsules can cause differentiation syndrome. Ask patients to immediately report any symptoms suggestive of differentiation syndrome, such as fever, cough or difficulty breathing, decreased urinary output, low blood pressure, rapid weight gain, or swelling of their arms or legs, to their healthcare provider for further evaluation [see Warnings and Precautions (5.2)].
Patients Without t(15;17) Translocation or PML/RARα Fusion
Advise patients that tretinoin capsules are not recommended for use in patients without t(15;17) translocation or PML/RARα fusion [see Warnings and Precautions (5.3)].
Leukocytosis
Inform patients that rapidly evolving leukocytosis, which can be life-threatening, can occur during treatment with tretinoin capsules [see Warnings and Precautions (5.4)].
Intracranial Hypertension
Advise patients that tretinoin capsules can cause intracranial hypertension, especially in pediatric patients. Ask patients to immediately report any symptoms suggestive of intracranial hypertension, such as headache, nausea, vomiting, and visual disturbances [see Warnings and Precautions (5.5)].
Lipid Abnormalities
Inform patients that hypercholesterolemia and/or hypertriglyceridemia can occur during treatment with tretinoin capsules. Advise patients on the need for monitoring fasting triglycerides and cholesterol [see Warnings and Precautions (5.6)].
Hepatotoxicity
Advise patients that tretinoin capsules can cause elevated liver function tests. Advise patients on the need for monitoring of liver function tests [see Warnings and Precautions (5.7)].
Thromboembolic Events
Inform patients that venous and arterial thromboembolic events, including cerebrovascular accident, myocardial infarction and renal infarct can occur during treatment with tretinoin capsules [see Warnings and Precautions (5.8)].
Lactation
Advise women not to breastfeed during treatment with tretinoin capsules and for 1 week after the last dose [see Use in Specific Populations (8.2)].
Administration Instructions
Advise patients to swallow tretinoin capsules whole with water. Advise patients not to chew, dissolve, or open capsules. Advise patients not to take a missed dose of tretinoin capsules unless it is more than 10 hours until the next scheduled dose. Advise patients that if vomiting occurs after tretinoin capsules administration, that they should not take an additional dose, but continue with the next scheduled dose [see Dosage and Administration (2.2)].
Effects on Ability to Drive and Use Machines
Advise patients that the ability to drive or operate machinery might be impaired when treated with tretinoin capsules, particularly if patients are experiencing dizziness or severe headache.
Manufactured for:
Endo USA
Malvern, PA 19355Made in France
© 2024 Endo, Inc. or one of its affiliates.
Revised: 07/2024
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL

NDC 10370-268-01
Tretinoin Capsules 10 mg
CAUSES BIRTH DEFECTS
DO NOT GET PREGNANT
Contains the active ingredient found in Vesanoid*
PHARMACIST: PLEASE DISPENSE WITH ATTACHED PATIENT INFORMATION LEAFLET.
Rx only
100 Capsules
PATIENT READ INFORMATION LEAFLET CAREFULLY
Each capsule contains:
Tretinoin................10 mgUSUAL DOSAGE: See package outsert for full prescribing information.
KEEP THIS AND ALL DRUGS OUT OF REACH OF CHILDREN.
Dispense in tight, light-resistant container. [see USP]
Store at 20ºC to 25ºC (68ºF to 77ºF). [see USP Controlled Room Temperature].
Protect from light.R08/2024
Manufactured for:
Endo USA
Malvern, PA 19355Made in France
*Vesanoid is a registered trademark of Roche
Laboratories Inc. Endo, Inc. is not affiliated with Roche Laboratories Inc. -
INGREDIENTS AND APPEARANCE
TRETINOIN
tretinoin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10370-268 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRETINOIN (UNII: 5688UTC01R) (TRETINOIN - UNII:5688UTC01R) TRETINOIN 10 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) EDETATE DISODIUM (UNII: 7FLD91C86K) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) NITROGEN (UNII: N762921K75) FERRIC OXIDE RED (UNII: 1K09F3G675) SOYBEAN OIL (UNII: 241ATL177A) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) ALCOHOL (UNII: 3K9958V90M) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) YELLOW WAX (UNII: 2ZA36H0S2V) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color yellow (Opaque Body) , brown (Opaque Cap) Score no score Shape CAPSULE Size 9mm Flavor Imprint Code TR Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10370-268-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/24/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201687 10/24/2012 Labeler - Endo USA, Inc. (119185057)

