Label: COMBIVENT RESPIMAT- ipratropium bromide and albuterol spray, metered
- NDC Code(s): 0597-0024-02
- Packager: Boehringer Ingelheim Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use COMBIVENT RESPIMAT safely and effectively. See full prescribing information for COMBIVENT RESPIMAT. COMBIVENT® RESPIMAT ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECOMBIVENT RESPIMAT is a combination of ipratropium bromide (an anticholinergic agent) and albuterol sulfate (a beta2-adrenergic agonist) indicated for use in patients with chronic obstructive ...
-
2 DOSAGE AND ADMINISTRATION The recommended dose of COMBIVENT RESPIMAT is one inhalation four times a day. Patients may take additional inhalations as required; however, the total number of inhalations should not exceed six ...
-
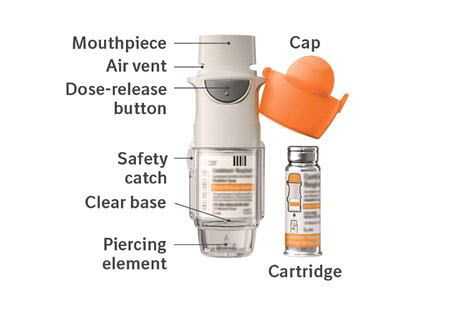
3 DOSAGE FORMS AND STRENGTHSCOMBIVENT RESPIMAT consists of a COMBIVENT RESPIMAT inhaler and an aluminum cylinder (COMBIVENT RESPIMAT cartridge) containing a combination of ipratropium bromide (as the monohydrate) and ...
-
4 CONTRAINDICATIONSCOMBIVENT RESPIMAT is contraindicated in the following conditions [see Warnings and Precautions (5.6)]: Hypersensitivity to any of the ingredients in COMBIVENT RESPIMAT - Hypersensitivity to ...
-
5 WARNINGS AND PRECAUTIONS5.1 Paradoxical Bronchospasm - COMBIVENT RESPIMAT can produce paradoxical bronchospasm that can be life-threatening. If it occurs, therapy with COMBIVENT RESPIMAT should be discontinued ...
-
6 ADVERSE REACTIONSUse of albuterol, a beta2-adrenergic agonist, may be associated with the following: Paradoxical bronchospasm [see Warnings and Precautions (5.1)] Cardiovascular effects [see Warnings and ...
-
7 DRUG INTERACTIONSCOMBIVENT RESPIMAT has been used concomitantly with other drugs, including beta-adrenergic bronchodilators, methylxanthines, and oral and inhaled steroids, commonly used in the treatment of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no randomized clinical studies of COMBIVENT RESPIMAT, or its individual components, ipratropium bromide and albuterol sulfate, in pregnant women ...
-
10 OVERDOSAGEThe effects of overdosage are expected to be related primarily to albuterol sulfate. Acute overdosage with ipratropium bromide by inhalation is unlikely since ipratropium bromide is not well ...
-
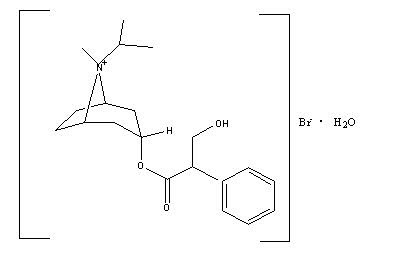
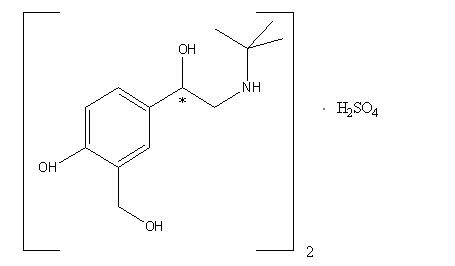
11 DESCRIPTIONCOMBIVENT RESPIMAT is a combination of ipratropium bromide (as the monohydrate) and albuterol sulfate. Ipratropium bromide is an anticholinergic bronchodilator chemically described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - COMBIVENT RESPIMAT: COMBIVENT RESPIMAT is a combination of the anticholinergic ipratropium bromide and the beta2-adrenergic agonist albuterol sulfate. The mechanisms ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Ipratropium bromide - Two-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic activity at doses up to ...
-
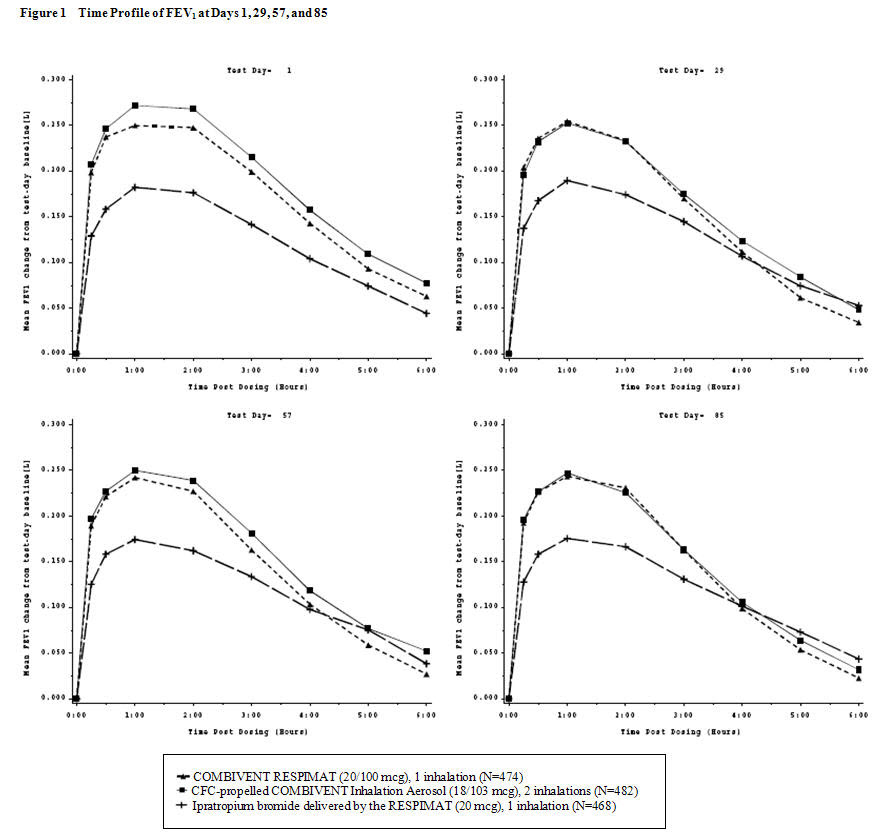
14 CLINICAL STUDIESThe efficacy of COMBIVENT RESPIMAT (20/100 mcg) was evaluated in COPD patients in one randomized, double-blind, double-dummy parallel group trial. This was a 12-week trial in a total of 1460 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCOMBIVENT RESPIMAT Inhalation Spray is supplied in a carton containing one COMBIVENT RESPIMAT cartridge and one COMBIVENT RESPIMAT inhaler. The COMBIVENT RESPIMAT cartridge is provided as an ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Paradoxical Bronchospasm - Inform patients that COMBIVENT RESPIMAT can produce paradoxical bronchospasm that ...
-
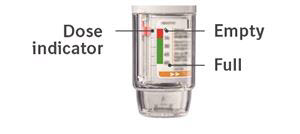
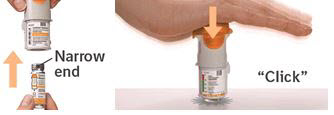
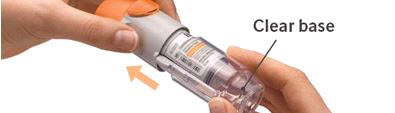
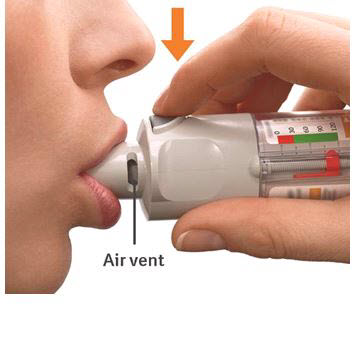
PATIENT PACKAGE INSERTInstructions for Use - COMBIVENT® RESPIMAT® (COM beh vent - RES peh mat) (ipratropium bromide and albuterol inhalation spray) For Oral Inhalation Only - Do not spray COMBIVENT RESPIMAT into your ...
-
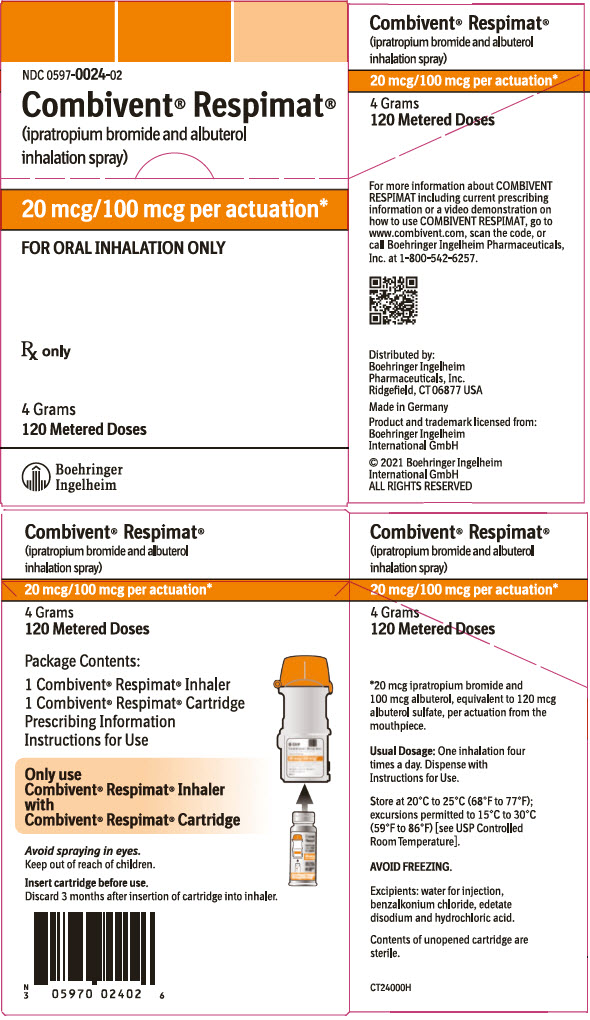
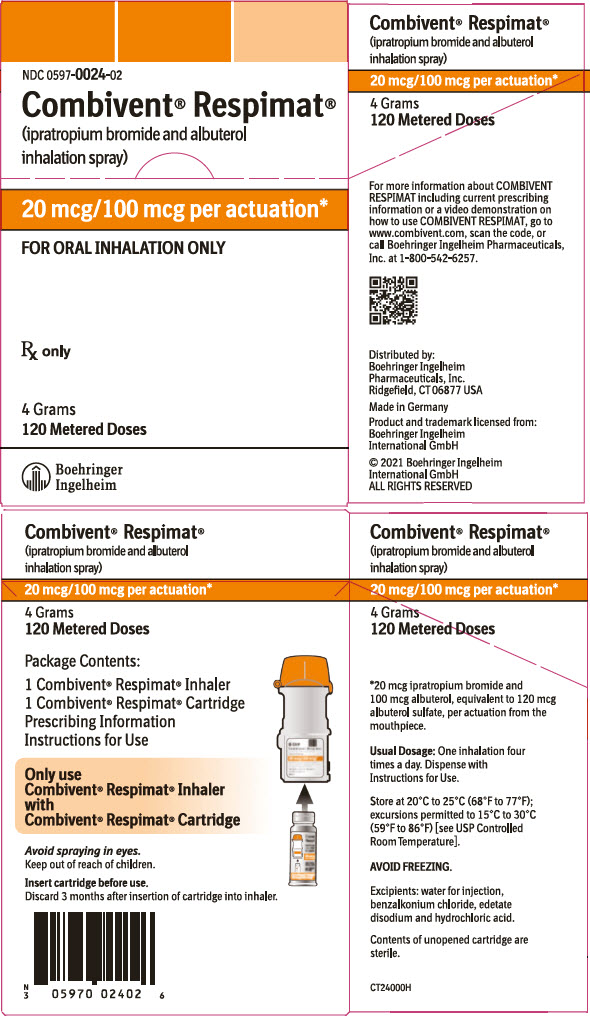
PRINCIPAL DISPLAY PANEL - 20 mcg/100 mcg CartonNDC 0597-0024-02 - Combivent® Respimat® (ipratropium bromide and albuterol - inhalation spray) 20 mcg/100 mcg per actuation* FOR ORAL INHALATION ONLY - Rx only - 4 Grams - 120 Metered Doses - Boehringer ...
-
INGREDIENTS AND APPEARANCEProduct Information