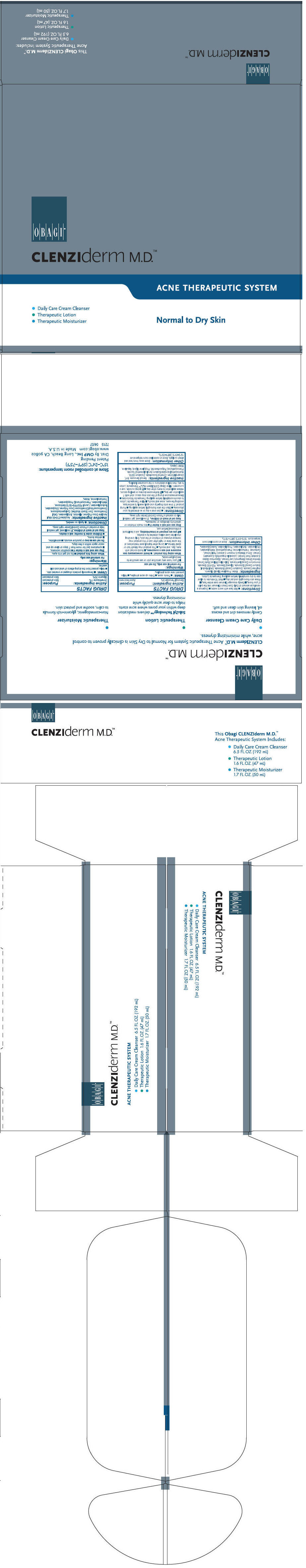
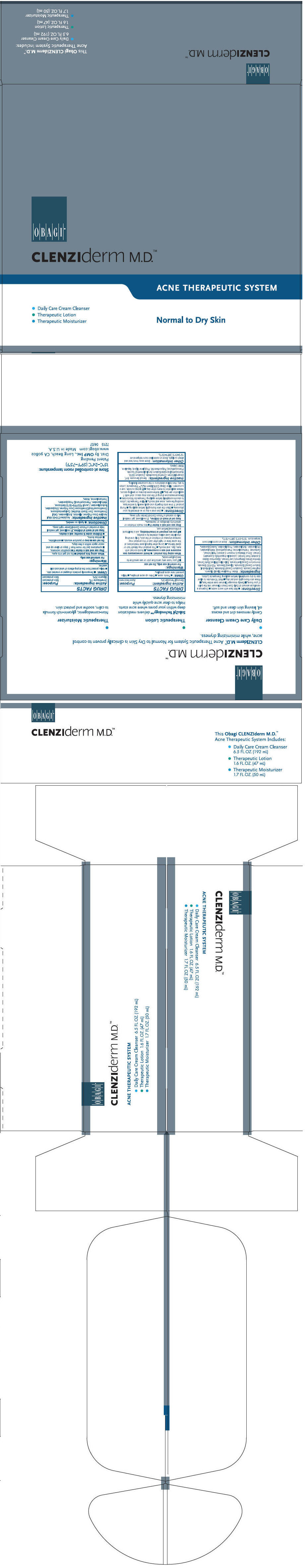
Label: CLENZIDERM NORMAL TO DRY SKIN ACNE THERAPEUTIC SYSTEM- glycerin and benzoyl peroxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 62032-113-65, 62032-114-70, 62032-512-03 - Packager: OMP, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 19, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Daily Care Cream Cleanser
- Directions
-
Ingredients
Water, Propylene Glycol, Glycerin, Arginine Cocoate, Disodium Cocoyl Glutamate, Cetyl Alcohol, Sodium Cocoyl Glutamate, Glyceryl Stearate, PEG-100 Stearate, Ammonium Cocoyl Isethionate, Salix Alba (Willow) Bark Extract, Mentha Citrata (Bergamot) Leaf Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Lavandula Angustifolia (Lavender) Extract, Citrus Medica Limonum (Lemon) Peel Extract, Carbomer, Triethanolamine, Phenoxyethanol, Methylparaben, Ethylparaben, Butylparaben, Propylparaben, Isobutylparaben.
- Other Information
- Therapeutic Lotion
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only. Do not use
- •
- If you have very sensitive skin or are sensitive to benzoyl peroxide.
When using this product
- •
- Avoid unneccessary sun exposure and use a sunscreen.
- •
- Avoid contact with eyes, lips, and mouth.
- •
- This product may bleach hair or dyed fabrics.
- •
- Using other topical acne medications at the same time or right after use of this product may increase dryness or irritation of the skin. Only one drug should be used unless directed by a doctor.
- •
- If you are pregnant or breast-feeding, ask a healthcare professional before use.
-
Directions
- •
- Use once a day or as directed by your physician.
- •
- Clean the skin thoroughly before applying.
- •
- Pump product 1 time onto fingertip and apply evenly to entire face, avoiding the eyes, nose and mouth.
- •
- Allow Therapeutic Lotion to absorb completely before applying Therapeutic Moisturizer.
- •
- Because excessive drying of the skin may occur, start with 1 application per day.
- •
- If bothersome dryness or peeling occurs, reduce application to every other day.
- •
- If going outside, use a sunscreen: Allow Obagi CLENZIderm M.D.™ Therapeutic Lotion to dry, then follow directions in the sunscreen labeling.
- Inactive Ingredients
- Other Information
- Therapeutic Moisturizer
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylate Cross Polymer, Allantoin, Butylparaben, Cetyl Dimethicone, Corn Starch Modified, Cyclopentasiloxane, Dimethicone/Vinyl Dimethicone Cross Polymer, Ethylparaben, Isobutylparaben, Lauryl PEG/PPG-18/18 Methicone, Metyhlparaben, Phenoxyethanol, Propylparaben, Triethanolamine, Water.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
CLENZIDERM NORMAL TO DRY SKIN ACNE THERAPEUTIC SYSTEM
glycerin and benzoyl peroxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62032-512 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62032-512-03 1 in 1 CARTON; Type 0: Not a Combination Product 07/01/2007 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 50 mL Part 2 1 BOTTLE, PLASTIC 47 mL Part 1 of 2 CLENZIDERM THERAPEUTIC MOISTURIZER
glycerin creamProduct Information Item Code (Source) NDC:62032-114 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) LAURYL PEG/PPG-18/18 METHICONE (UNII: ZJ5S27D9NX) DIMETHICONE (UNII: 92RU3N3Y1O) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) BUTYLPARABEN (UNII: 3QPI1U3FV8) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62032-114-70 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 07/01/2007 Part 2 of 2 CLENZIDERM THERAPEUTIC
benzoyl peroxide lotionProduct Information Item Code (Source) NDC:62032-113 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL BENZOATE (UNII: N863NB338G) DICAPRYLYL ETHER (UNII: 77JZM5516Z) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SQUALANE (UNII: GW89575KF9) POLYSORBATE 60 (UNII: CAL22UVI4M) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM (UNII: 7FLD91C86K) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62032-113-65 47 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 07/01/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 07/01/2007 Labeler - OMP, INC. (790553353) Establishment Name Address ID/FEI Business Operations Ei INC. 105803274 MANUFACTURE(62032-512) , LABEL(62032-512) , PACK(62032-512) , ANALYSIS(62032-512) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 MANUFACTURE(62032-512)