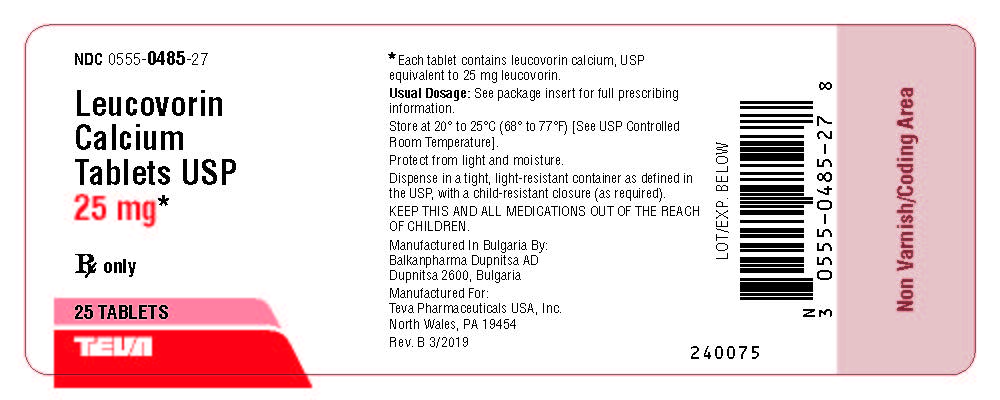
Label: LEUCOVORIN CALCIUM tablet
- NDC Code(s): 0555-0484-01, 0555-0484-02, 0555-0485-27
- Packager: Teva Pharmaceuticals USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 1, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Leucovorin Calcium Tablets USP contain either 5 mg or 25 mg leucovorin as the calcium salt of N-[4-[[(2-amino-5-formyl-1, 4, 5, 6, 7, 8-hexahydro-4-oxo-6-pteridinyl)methyl] amino]benzoyl]-L-glutamic acid. This is equivalent to 5.4 mg or 27.01 mg of anhydrous leucovorin calcium, respectively. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The 25 mg tablet also contains D&C yellow no. 10 aluminum lake and FD&C blue no. 1 aluminum lake.
Leucovorin is a water soluble form of reduced folate in the folate group; it is useful as an antidote to drugs which act as folic acid antagonists. These tablets are intended for oral administration only.
The structural formula is as follows:
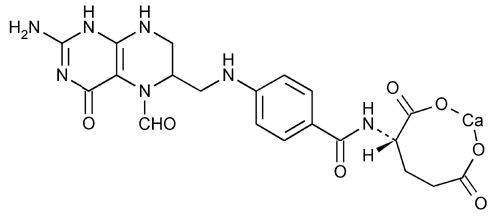
C20H21CaN7O7 M.W. 511.5
-
CLINICAL PHARMACOLOGY
Leucovorin is a racemic mixture of the diastereoisomers of the 5-formyl derivative of tetrahydrofolic acid. The biologically active compound of the mixture is the (-)-L-isomer, known as Citrovorum factor, or (-)-folinic acid. Leucovorin does not require reduction by the enzyme dihydrofolate reductase in order to participate in reactions utilizing folates as a source of “one-carbon” moieties. Following oral administration, leucovorin is rapidly absorbed and enters the general body pool of reduced folates. The increase in plasma and serum folate activity (determined microbiologically with Lactobacillus casei) seen after oral administration of leucovorin is predominantly due to 5-methyltetrahydrofolate.
Twenty normal men were given a single, oral 15 mg dose (7.5 mg/m2) of leucovorin calcium and serum folate concentrations were assayed with L. casei. Mean values observed (± one standard error) were:
- Time to peak serum folate concentration: 1.72 ± 0.08 hours,
- Peak serum folate concentration achieved: 268 ± 18 ng/mL,
- Serum folate half-disappearance time: 3.5 hours.
Oral tablets yielded areas under the serum folate concentration-time curves (AUCs) that were 12% greater than equal amounts of leucovorin given intramuscularly and equal to the same amounts given intravenously.
Oral absorption of leucovorin is saturable at doses above 25 mg. The apparent bioavailability of leucovorin was 97% for 25 mg, 75% for 50 mg and 37% for 100 mg.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
In the treatment of accidental overdosage of folic acid antagonists, leucovorin should be administered as promptly as possible. As the time interval between antifolate administration (e.g., methotrexate) and leucovorin rescue increases, leucovorin’s effectiveness in counteracting hematologic toxicity decreases.
Monitoring of the serum methotrexate concentration is essential in determining the optimal dose and duration of treatment with leucovorin.
Delayed methotrexate excretion may be caused by a third space fluid accumulation (i.e., ascites, pleural effusion), renal insufficiency, or inadequate hydration. Under such circumstances, higher doses of leucovorin or prolonged administration may be indicated. Doses higher than those recommended for oral use must be given intravenously.
Leucovorin may enhance the toxicity of fluorouracil. Deaths from severe enterocolitis, diarrhea, and dehydration have been reported in elderly patients receiving weekly leucovorin and fluorouracil.1 Concomitant granulocytopenia and fever were present in some but not all of the patients.
The concomitant use of leucovorin with trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection was associated with increased rates of treatment failure and mortality in a placebo-controlled study.
-
PRECAUTIONS
General
Parenteral administration is preferable to oral dosing if there is a possibility that the patient may vomit or not absorb the leucovorin. Leucovorin has no effect on other established toxicities of methotrexate such as the nephrotoxicity resulting from drug and/or metabolite precipitation in the kidney.
Drug Interactions
Folic acid in large amounts may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone, and increase the frequency of seizures in susceptible children.
Preliminary animal and human studies have shown that small quantities of systemically administered leucovorin enter CSF primarily as 5-methyltetrahydrofolate and, in humans, remain 1 to 3 orders of magnitude lower than usual methotrexate concentrations following intrathecal administration. However, high doses of leucovorin may reduce the efficacy of intrathecally administered methotrexate.
Leucovorin may enhance the toxicity of fluorouracil (see WARNINGS).
-
ADVERSE REACTIONS
Allergic sensitization, including anaphylactoid reactions and urticaria, has been reported following the administration of both oral and parenteral leucovorin.
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals USA, Inc. at 1-888-838-2872 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Leucovorin calcium tablets are intended for oral administration. Because absorption is saturable, oral administration of doses greater than 25 mg is not recommended.
Impaired Methotrexate Elimination or Inadvertent Overdosage
Leucovorin rescue should begin as soon as possible after an inadvertent overdosage and within 24 hours of methotrexate administration when there is delayed excretion (see WARNINGS). Leucovorin 15 mg (10 mg/m2) should be administered IM, IV, or PO every 6 hours until serum methotrexate level is less than 10 -8 M. In the presence of gastrointestinal toxicity, nausea, or vomiting, leucovorin should be administered parenterally.
Serum creatinine and methotrexate levels should be determined at 24 hour intervals. If the 24 hour serum creatinine has increased 50% over baseline or if the 24 hour methotrexate level is greater than 5 x 10 -6 M or the 48 hour level is greater than 9 x 10 -7 M, the dose of leucovorin should be increased to 150 mg (100 mg/m2) IV every 3 hours until the methotrexate level is less than 10 -8 M. Doses greater than 25 mg should be given parenterally (see CLINICAL PHARMACOLOGY).
Hydration (3 L/d) and urinary alkalinization with sodium bicarbonate should be employed concomitantly. The bicarbonate dose should be adjusted to maintain the urine pH at 7 or greater.
The recommended dose of leucovorin to counteract hematologic toxicity from folic acid antagonists with less affinity for mammalian dihydrofolate reductase than methotrexate (i.e., trimethoprim, pyrimethamine) is substantially less, and 5 to 15 mg of leucovorin per day has been recommended by some investigators.
Patients who experience delayed early methotrexate elimination are likely to develop reversible non-oliguric renal failure. In addition to appropriate leucovorin therapy, these patients require continuing hydration and urinary alkalinization, and close monitoring of fluid and electrolyte status, until the serum methotrexate level has fallen to below 0.05 micromolar and the renal failure has resolved.
Some patients will have abnormalities in methotrexate elimination or renal function following methotrexate administration, which are significant but less severe. These abnormalities may or may not be associated with significant clinical toxicity. If significant clinical toxicity is observed, leucovorin rescue should be extended for an additional 24 hours (total 14 doses over 84 hours) in subsequent courses of therapy. The possibility that the patient is taking other medications which interact with methotrexate (e.g., medications which may interfere with methotrexate elimination or binding to serum albumin) should always be reconsidered when laboratory abnormalities or clinical toxicities are observed.
-
HOW SUPPLIED
Leucovorin Calcium Tablets USP, 5 mg are available as white, round, unscored, biconvex tablets, debossed with b on one side and 484 on the other side, packaged in bottles of 30 (NDC 0555-0484-01), 100 (NDC 0555-0484-02) and 1000 (NDC 0555-0484-05) tablets.
Leucovorin Calcium Tablets USP, 25 mg are available as pale green, round, unscored, biconvex tablets, debossed with b on one side and 485 on the other side, packaged in bottles of 25 (NDC 0555-0485-27) and 500 (NDC 0555-0485-04) tablets.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant
closure (as required).
Protect from light and moisture.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
-
REFERENCES
- Grem JL, Shoemaker DD, Petrelli NJ, Douglas HO. Severe and fatal toxic effects observed in treatment with high- and low-dose leucovorin plus 5-fluorouracil for colorectal carcinoma. Cancer Treat Rep 1987;71:1122.
- Link MP, Goorin AM, Miser AW et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986;314:1600-1606.
Manufactured In Bulgaria By:
Balkanpharma Dupnitsa AD
Dupnitsa 2600, Bulgaria
Manufactured For:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454
Rev. B 3/2019
- Package/Label Display Panel
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
LEUCOVORIN CALCIUM
leucovorin calcium tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0555-0484 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEUCOVORIN CALCIUM (UNII: RPR1R4C0P4) (LEUCOVORIN - UNII:Q573I9DVLP) LEUCOVORIN 5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape ROUND Size 8mm Flavor Imprint Code b;484 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0555-0484-01 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/30/1990 2 NDC:0555-0484-02 100 in 1 BOTTLE; Type 0: Not a Combination Product 06/30/1990 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA071198 06/30/1990 LEUCOVORIN CALCIUM
leucovorin calcium tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0555-0485 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEUCOVORIN CALCIUM (UNII: RPR1R4C0P4) (LEUCOVORIN - UNII:Q573I9DVLP) LEUCOVORIN 25 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) Product Characteristics Color green (pale green) Score no score Shape ROUND Size 8mm Flavor Imprint Code b;485 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0555-0485-27 25 in 1 BOTTLE; Type 0: Not a Combination Product 06/30/1990 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA071199 06/30/1990 Labeler - Teva Pharmaceuticals USA, Inc. (001627975)