Label: CLOPIDOGREL BISULFATE tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 43353-269-30, 43353-269-60 - Packager: Aphena Pharma Solutions - Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 60429-301
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 17, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CLOPIDOGREL TABLETS safely and effectively. See full prescribing information for CLOPIDOGREL TABLETS.
CLOPIDOGREL tablets, for oral use
Initial U.S. Approval: 1997WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
See full prescribing information for complete boxed warning.
- •
- Effectiveness of clopidogrel bisulfate depends on conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. (5.1, 12.3)
- •
- Tests are available to identify patients who are CYP2C19 poor metabolizers. (12.5)
- •
- Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers. (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Clopidogrel bisulfate is a P2Y12 platelet inhibitor indicated for:
- 2.
- Acute coronary syndrome
- For patients with non-ST-segment elevation ACS [unstable angina (UA)/non-ST-elevation myocardial infarction (NSTEMI)], clopidogrel bisulfate has been shown to reduce the rate of myocardial infarction (MI) and stroke. (1.1)
- For patients with ST-elevation myocardial infarction (STEMI), clopidogrel bisulfate has been shown to reduce the rate of MI and stroke. (1.1)
- 2.
- Recent MI, recent stroke, or established peripheral arterial disease. Clopidogrel bisulfate has been shown to reduce the rate of MI and stroke. (1.2)
DOSAGE AND ADMINISTRATION
- •
- Acute coronary syndrome (2.1)
- 3.
- Initiate clopidogrel with a single 300-mg oral loading dose and then continue at 75 mg once daily
- 4.
- Initiating clopidogrel without a loading dose will delay establishment of an antiplatelet effect by several days
- •
- Recent MI, recent stroke, or established peripheral arterial disease: 75 mg once daily orally without a loading dose (2.2)
DOSAGE FORMS AND STRENGTHS
Tablets: 75 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- •
- CYP2C19 inhibitors: Avoid concomitant use of omeprazole or esomeprazole. (5.1)
- •
- Bleeding: Clopidogrel bisulfate increases risk of bleeding. (5.2)
- •
- Discontinuation: Premature discontinuation increases risk of cardiovascular events. Discontinue 5 days prior to elective surgery that has a major risk of bleeding. (5.3)
- •
- Thrombotic thrombocytopenic purpura (TTP) has been reported. (5.4)
- •
- Cross-reactivity among thienopyridines has been reported. (5.5)
ADVERSE REACTIONS
Bleeding, including life-threatening and fatal bleeding, is the most commonly reported adverse reaction. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Apotex Inc. at 1-800-706-5575 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Nursing mothers: Discontinue drug or nursing. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
1 INDICATIONS AND USAGE
1.1 Acute Coronary Syndrome (ACS)
1.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease
2 DOSAGE AND ADMINISTRATION
2.1 Acute Coronary Syndrome
2.2 Recent MI, Recent Stroke, or Established Peripheral Arterial Disease
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Active Bleeding
4.2 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Diminished Antiplatelet Activity in Patients with Impaired CYP2C19 Function
5.2 General Risk of Bleeding
5.3 Discontinuation of Clopidogrel Bisulfate
5.4 Thrombotic Thrombocytopenic Purpura (TTP)
5.5 Cross-Reactivity among Thienopyridines
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP2C19 Inhibitors
7.2 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
7.3 Warfarin (CYP2C9 Substrates)
7.4 SSRIs and SNRIs
7.5 Repaglinide (CYP2C8 Substrates)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Acute Coronary Syndrome
14.2 Recent Myocardial Infarction, Recent Stroke, or Established Peripheral Arterial Disease
14.3 No Demonstrated Benefit of Clopidogrel plus Aspirin in Patients with Multiple Risk Factors or Established Vascular Disease
16 HOW SUPPLIED/STORAGE AND HANDLING
Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of clopidogrel bisulfate results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19 [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)]. Clopidogrel bisulfate at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed "CYP2C19 poor metabolizers"). Tests are available to identify patients who are CYP2C19 poor metabolizers [see Clinical Pharmacology (12.5)]. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
-
1 INDICATIONS AND USAGE
1.1 Acute Coronary Syndrome (ACS)
- •
- Clopidogrel tablets are indicated to reduce the rate of myocardial infarction and stroke (MI) in patients with non-ST-segment elevation ACS [unstable angina (UA)/non-ST-elevation myocardial infarction (NSTEMI)], including patients who are to be managed medically and those who are to be managed with coronary revascularization. Clopidogrel should be administered in conjunction with aspirin.
- •
- Clopidogrel tablets are indicated to reduce the rate of myocardial infarction and stroke in patients with acute ST-elevation myocardial infarction (STEMI) who are to be managed medically. Clopidogrel bisulfate should be administered in conjunction with aspirin.
-
2 DOSAGE AND ADMINISTRATION
2.1 Acute Coronary Syndrome
In patients who need an antiplatelet effect within hours, initiate clopidogrel tablets with a single 300-mg oral loading dose and then continue at 75 mg once daily. Initiating clopidogrel without a loading dose will delay establishment of an antiplatelet effect by several days [see Clinical Pharmacology (12.3) and Clinical Studies (14.1)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Active Bleeding
Clopidogrel bisulfate is contraindicated in patients with active pathological bleeding such as peptic ulcer or intracranial hemorrhage.
4.2 Hypersensitivity
Clopidogrel bisulfate is contraindicated in patients with hypersensitivity (e.g., anaphylaxis) to clopidogrel or any component of the product [see Adverse Reactions (6.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Diminished Antiplatelet Activity in Patients with Impaired CYP2C19 Function
Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is achieved through an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by genetic variations in CYP2C19 [see Boxed Warning].
The metabolism of clopidogrel can also be impaired by drugs that inhibit CYP2C19, such as omeprazole or esomeprazole. Avoid concomitant use of clopidogrel with omeprazole or esomeprazole because both significantly reduce the antiplatelet activity of clopidogrel [see Drug Interactions (7.1)].
5.2 General Risk of Bleeding
Thienopyridines, including clopidogrel bisulfate, increase the risk of bleeding.
Thienopyridines inhibit platelet aggregation for the lifetime of the platelet (7 to 10 days). Because the half-life of clopidogrel’s active metabolite is short, it may be possible to restore hemostasis by administering exogenous platelets; however, platelet transfusions within 4 hours of the loading dose or 2 hours of the maintenance dose may be less effective.
5.3 Discontinuation of Clopidogrel Bisulfate
Discontinuation of clopidogrel bisulfate increases the risk of cardiovascular events. If clopidogrel must be temporarily discontinued (e.g., to treat bleeding or for surgery with a major risk of bleeding), restart it as soon as possible. When possible, interrupt therapy with clopidogrel for five days prior to such surgery. Resume clopidogrel as soon as hemostasis is achieved.
5.4 Thrombotic Thrombocytopenic Purpura (TTP)
TTP, sometimes fatal, has been reported following use of clopidogrel bisulfate, sometimes after a short exposure (<2 weeks). TTP is a serious condition that requires urgent treatment including plasmapheresis (plasma exchange). It is characterized by thrombocytopenia, microangiopathic hemolytic anemia (schistocytes [fragmented RBCs] seen on peripheral smear), neurological findings, renal dysfunction, and fever [see Adverse Reactions (6.2)].
5.5 Cross-Reactivity among Thienopyridines
Hypersensitivity including rash, angioedema or hematologic reaction have been reported in patients receiving clopidogrel, including patients with a history of hypersensitivity or hematologic reaction to other thienopyridines [see Contraindications (4.2) and Adverse Reactions (6.2)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in the labeling:
- •
- Bleeding [see Warnings and Precautions (5.2)]
- •
- Thrombotic thrombocytopenic purpura [see Warnings and Precautions (5.4)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions and durations of follow up, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clopidogrel bisulfate has been evaluated for safety in more than 54,000 patients, including over 21,000 patients treated for one year or more. The clinically important adverse reactions observed in trials comparing clopidogrel plus aspirin to placebo plus aspirin and trials comparing clopidogrel bisulfate alone to aspirin alone are discussed below.
Bleeding
CURE
In CURE, clopidogrel bisulfate use with aspirin was associated with an increase in major bleeding (primarily gastrointestinal and at puncture sites) compared to placebo with aspirin (see Table 1). The incidence of intracranial hemorrhage (0.1%) and fatal bleeding (0.2%) were the same in both groups. Other bleeding events that were reported more frequently in the clopidogrel group were epistaxis, hematuria, and bruise.
The overall incidence of bleeding is described in Table 1.
Table 1: CURE Incidence of Bleeding Complications (% patients)
Event
Clopidogrel Bisulfate
(+aspirin)
(n=6259)Placebo
(+ aspirin)
(n=6303)Major bleeding †
3.7
2.7
Life-threatening bleeding
2.2
1.8
Fatal
0.2
0.2
5 g/dL hemoglobin drop
0.9
0.9
Requiring surgical intervention
0.7
0.7
Hemorrhagic strokes
0.1
0.1
Requiring inotropes
0.5
0.5
Requiring transfusion (>4 units)
1.2
1.0
Other major bleeding
1.6
1.0
Significantly disabling
0.4
0.3
Intraocular bleeding with significant loss of vision
0.05
0.03
Requiring 2 to 3 units of blood
1.3
0.9
Minor bleeding ¶
5.1
2.4
† Life-threatening and other major bleeding.
¶ Led to interruption of study medication.COMMIT
In COMMIT, similar rates of major bleeding were observed in the clopidogrel bisulfate and placebo groups, both of which also received aspirin (see Table 2).
Table 2: Incidence of Bleeding Events in COMMIT (% patients)
Type of bleeding
Clopidogrel bisulfate
(+ aspirin)
(n=22961)Placebo
(+ aspirin)
(n=22891)p-value
Major* noncerebral or cerebral bleeding
0.6
0.5
0.59
Major noncerebral
0.4
0.3
0.48
Fatal
0.2
0.2
0.90
Hemorrhagic stroke
0.2
0.2
0.91
Fatal
0.2
0.2
0.81
Other noncerebral bleeding (non-major)
3.6
3.1
0.005
Any noncerebral bleeding
3.9
3.4
0.004
* Major bleeds were cerebral bleeds or non-cerebral bleeds thought to have caused death or that required transfusion.
CAPRIE (Clopidogrel bisulfate vs. Aspirin)
In CAPRIE, gastrointestinal hemorrhage occurred at a rate of 2.0% in those taking clopidogrel bisulfate vs. 2.7% in those taking aspirin; bleeding requiring hospitalization occurred in 0.7% and 1.1%, respectively. The incidence of intracranial hemorrhage was 0.4% for clopidogrel bisulfate compared to 0.5% for aspirin.
Other bleeding events that were reported more frequently in the clopidogrel bisulfate group were epistaxis and hematoma.
Other Adverse Events
In CURE and CHARISMA, which compared clopidogrel bisulfate plus aspirin to aspirin alone, there was no difference in the rate of adverse events (other than bleeding) between clopidogrel bisulfate and placebo.
In CAPRIE, which compared clopidogrel bisulfate to aspirin, pruritus was more frequently reported in those taking clopidogrel bisulfate. No other difference in the rate of adverse events (other than bleeding) was reported.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of clopidogrel bisulfate. Because these reactions are reported voluntarily from a population of an unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hemorrhages, including those with fatal outcome, have been reported in patients treated with clopidogrel.
- •
- Blood and lymphatic system disorders: Agranulocytosis, aplastic anemia/pancytopenia, thrombotic thrombocytopenic purpura (TTP), acquired hemophilia A
- •
- Gastrointestinal disorders: Colitis (including ulcerative or lymphocytic colitis), pancreatitis, stomatitis, gastric/duodenal ulcer, diarrhea
- •
- General disorders and administration site condition: Fever
- •
- Hepato-biliary disorders: Acute liver failure, hepatitis (non-infectious), abnormal liver function test
- •
- Immune system disorders: Hypersensitivity reactions, anaphylactoid reactions, serum sickness
- •
- Musculoskeletal, connective tissue and bone disorders: Myalgia, arthralgia, arthritis
- •
- Nervous system disorders: Taste disorders, headache
- •
- Psychiatric disorders: Confusion, hallucinations
- •
- Respiratory, thoracic and mediastinal disorders: Bronchospasm, interstitial pneumonitis, eosinophilic pneumonia
- •
- Renal and urinary disorders: Increased creatinine levels
- •
- Skin and subcutaneous tissue disorders: Maculopapular, erythematous or exfoliative rash, urticaria, bullous dermatitis, eczema, toxic epidermal necrolysis, Stevens-Johnson syndrome, acute generalized exanthematous pustulosis (AGEP), angioedema, drug-induced hypersensitivity syndrome, drug rash with eosinophilia and systemic symptoms (DRESS), erythema multiforme, lichen planus, generalized pruritus
- •
- Vascular disorders: Vasculitis, hypotension
-
7 DRUG INTERACTIONS
7.1 CYP2C19 Inhibitors
Clopidogrel is metabolized to its active metabolite in part by CYP2C19. Concomitant use of drugs that inhibit the activity of this enzyme results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition [see Warnings and Precautions (5.1)].
Omeprazole or esomeprazole
Avoid concomitant use of clopidogrel bisulfate with omeprazole or esomeprazole. In clinical studies, omeprazole was shown to reduce significantly the antiplatelet activity of clopidogrel bisulfate when given concomitantly or 12 hours apart. A similar reduction in antiplatelet activity was observed with esomeprazole when given concomitantly with clopidogrel bisulfate. Dexlansoprazole, lansoprazole and pantoprazole had less effect on the antiplatelet activity of clopidogrel bisulfate than did omeprazole or esomeprazole [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)].
7.2 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Coadministration of clopidogrel bisulfate and NSAIDs increases the risk of gastrointestinal bleeding.
7.3 Warfarin (CYP2C9 Substrates)
Although the administration of clopidogrel 75 mg per day did not modify the pharmacokinetics of S-warfarin (a CYP2C9 substrate) or INR in patients receiving long-term warfarin therapy, coadministration of clopidogrel bisulfate with warfarin increases the risk of bleeding because of independent effects on hemostasis.
However, at high concentrations in vitro, clopidogrel inhibits CYP2C9.
7.4 SSRIs and SNRIs
Since selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) affect platelet activation, the concomitant administration of SSRIs and SNRIs with clopidogrel may increase the risk of bleeding.
7.5 Repaglinide (CYP2C8 Substrates)
The acyl-β-glucuronide metabolite of clopidogrel is a strong inhibitor of CYP2C8. Clopidogrel can increase the systemic exposure to drugs that are primarily cleared by CYP2C8, thereby needing dose-adjustment and/or appropriate monitoring.
Concomitant administration of clopidogrel with repaglinide significantly increases systemic exposures to repaglinide [see Clinical Pharmacology (12.3)]. When concomitant use is required in a patient maintained on clopidogrel, initiate repaglinide at 0.5 mg with each meal and titrate based on blood glucose levels. Do not exceed a total daily dose of 4 mg. If concomitant use of clopidogrel is required in a patient stabilized on higher doses of repaglinide, down titrate the dose of repaglinide based on blood glucose levels to not exceed a total daily dose of 4 mg.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Reproduction studies performed in rats and rabbits at doses up to 500 and 300 mg/kg/day, respectively (65 and 78 times the recommended daily human dose, respectively, on a mg/m2 basis), revealed no evidence of impaired fertility or fetotoxicity due to clopidogrel. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of a human response, clopidogrel bisulfate should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
Studies in rats have shown that clopidogrel and/or its metabolites are excreted in the milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from clopidogrel, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness in pediatric populations have not been established.
Additional information describing a clinical study in which efficacy was not demonstrated in neonates and infants is approved in the package insert for Bristol-Myers Squibb’s clopidogrel tablets. However, due to Bristol-Myers Squibb’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
8.5 Geriatric Use
Of the total number of subjects in the CAPRIE and CURE controlled clinical studies, approximately 50% of patients treated with clopidogrel bisulfate were 65 years of age and older, and 15% were 75 years and older. In COMMIT, approximately 58% of the patients treated with clopidogrel bisulfate were 60 years and older, 26% of whom were 70 years and older.
The observed risk of bleeding events with clopidogrel plus aspirin versus placebo plus aspirin by age category is provided in Table 1 and Table 2 for the CURE and COMMIT trials, respectively [see Adverse Reactions (6.1)]. No dosage adjustment is necessary in elderly patients.
8.6 Renal Impairment
Experience is limited in patients with severe and moderate renal impairment [see Clinical Pharmacology (12.2)].
8.7 Hepatic Impairment
No dosage adjustment is necessary in patients with hepatic impairment [see Clinical Pharmacology (12.2)].
-
10 OVERDOSAGE
Platelet inhibition by clopidogrel is irreversible and will last for the life of the platelet. Overdose following clopidogrel administration may result in bleeding complications. A single oral dose of clopidogrel at 1,500 or 2,000 mg/kg was lethal to mice and to rats and at 3,000 mg/kg to baboons. Symptoms of acute toxicity were vomiting, prostration, difficult breathing, and gastrointestinal hemorrhage in animals.
Based on biological plausibility, platelet transfusion may restore clotting ability.
-
11 DESCRIPTION
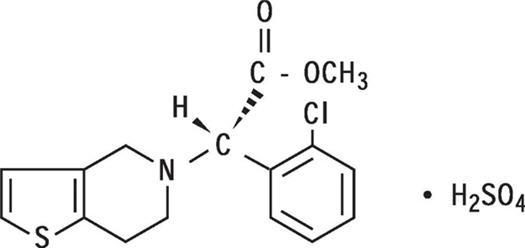
Clopidogrel bisulfate is an inhibitor of ADP-induced platelet aggregation acting by direct inhibition of adenosine diphosphate (ADP) binding to its receptor and of the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex. Chemically it is methyl (+)-(S)-α-(2-chlorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-acetate sulfate (1:1). The empirical formula of clopidogrel bisulfate is C16H16ClNO2S•H2SO4 and its molecular weight is 419.9.
The structural formula is as follows:
Clopidogrel bisulfate is a white to off-white powder. It is practically insoluble in water at neutral pH but freely soluble at pH 1. It also dissolves freely in methanol, dissolves sparingly in methylene chloride, and is practically insoluble in ethyl ether. It has a specific optical rotation of about +56°.
Clopidogrel tablets, USP 75 mg for oral administration are provided as pink, round, biconvex, film coated tablets, engraved “APO” on one side, “CL” over “75” on the other side. The tablets contain 97.875 mg of clopidogrel bisulfate, which is the molar equivalent of 75 mg of clopidogrel base.
Clopidogrel tablets 300 mg for oral administration are provided as pink, oblong, biconvex, film coated tablets engraved "APO" on one side and "CL 300" on the other side. The tablets contain 392.0 mg of clopidogrel bisulfate, which is the molar equivalent of 300 mg of clopidogrel base.
Each tablet contains anhydrous lactose, colloidal silicon dioxide, crospovidone, methylcellulose and zinc stearate as inactive ingredients. The pink film coating contains hydroxypropyl cellulose, hypromellose, polyethylene glycol, red ferric oxide and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clopidogrel is an inhibitor of platelet activation and aggregation through the irreversible binding of its active metabolite to the P2Y12 class of ADP receptors on platelets.
12.2 Pharmacodynamics
Clopidogrel must be metabolized by CYP450 enzymes to produce the active metabolite that inhibits platelet aggregation. The active metabolite of clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet P2Y12 receptor and the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation. This action is irreversible. Consequently, platelets exposed to clopidogrel's active metabolite are affected for the remainder of their lifespan (about 7 to 10 days). Platelet aggregation induced by agonists other than ADP is also inhibited by blocking the amplification of platelet activation by released ADP.
Dose-dependent inhibition of platelet aggregation can be seen 2 hours after single oral doses of clopidogrel. Repeated doses of 75 mg clopidogrel per day inhibit ADP-induced platelet aggregation on the first day, and inhibition reaches steady state between Day 3 and Day 7. At steady state, the average inhibition level observed with a dose of 75 mg clopidogrel per day was between 40% and 60%. Platelet aggregation and bleeding time gradually return to baseline values after treatment is discontinued, generally in about 5 days.
Geriatric Patients
Elderly (≥75 years) and young healthy subjects had similar effects on platelet aggregation.
Renally-Impaired Patients
After repeated doses of 75 mg clopidogrel per day, patients with severe renal impairment (creatinine clearance from 5 to 15 mL/min) and moderate renal impairment (creatinine clearance from 30 to 60 mL/min) showed low (25%) inhibition of ADP-induced platelet aggregation.
Hepatically-Impaired Patients
After repeated doses of 75 mg clopidogrel per day for 10 days in patients with severe hepatic impairment, inhibition of ADP-induced platelet aggregation was similar to that observed in healthy subjects.
Gender
In a small study comparing men and women, less inhibition of ADP-induced platelet aggregation was observed in women.
12.3 Pharmacokinetics
Clopidogrel is a prodrug and is metabolized to a pharmacologically active metabolite and inactive metabolites.
Absorption
After single and repeated oral doses of 75 mg per day, clopidogrel is rapidly absorbed. Absorption is at least 50%, based on urinary excretion of clopidogrel metabolites.
Effect of Food
Clopidogrel can be administered with or without food. In a study in healthy male subjects when clopidogrel 75 mg per day was given with a standard breakfast, mean inhibition of ADP-induced platelet aggregation was reduced by less than 9%. The active metabolite AUC0–24 was unchanged in the presence of food, while there was a 57% decrease in active metabolite Cmax. Similar results were observed when a clopidogrel 300 mg loading dose was administered with a high-fat breakfast.
Metabolism
Clopidogrel is extensively metabolized by two main metabolic pathways: one mediated by esterases and leading to hydrolysis into an inactive carboxylic acid derivative (85% of circulating metabolites) and one mediated by multiple cytochrome P450 enzymes. Cytochromes first oxidize clopidogrel to a 2-oxo-clopidogrel intermediate metabolite. Subsequent metabolism of the 2-oxo-clopidogrel intermediate metabolite results in formation of the active metabolite, a thiol derivative of clopidogrel. The active metabolite is formed mostly by CYP2C19 with contributions from several other CYP enzymes, including CYP1A2, CYP2B6 and CYP3A. The active thiol metabolite binds rapidly and irreversibly to platelet receptors, thus inhibiting platelet aggregation for the lifespan of the platelet.
The Cmax of the active metabolite is twice as high following a single 300 mg clopidogrel loading dose as it is after four days of 75 mg maintenance dose. Cmax occurs approximately 30 to 60 minutes after dosing. In the 75 to 300 mg dose range, the pharmacokinetics of the active metabolite deviates from dose proportionality: 4-fold the dose results in 2.0- and 2.7-fold the Cmax and AUC, respectively.
Elimination
Following an oral dose of 14C-labeled clopidogrel in humans, approximately 50% of total radioactivity was excreted in urine and approximately 46% in feces over the 5 days post-dosing. After a single, oral dose of 75 mg, clopidogrel has a half-life of approximately 6 hours. The half-life of the active metabolite is about 30 minutes.
Drug Interactions
Effect of other drugs on Clopidogrel
Clopidogrel is metabolized to its active metabolite in part by CYP2C19. Concomitant use of certain inhibitors of this enzyme results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition.
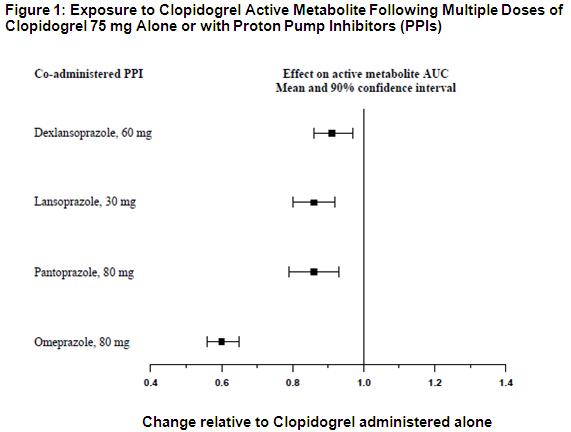
Proton Pump Inhibitors (PPI)
The effect of proton pump inhibitors (PPI) on the systemic exposure to the clopidogrel active metabolite following multiple doses of clopidogrel 75 mg evaluated in dedicated drug interaction studies is presented in Figure 1.
Pharmacodynamic and pharmacokinetic parameters measured in these studies showed that the interaction was highest with omeprazole and least with dexlansoprazole.
Effect of clopidogrel on other drugs
In vitro studies have shown that the glucuronide metabolite of clopidogrel is a strong inhibitor of CYP2C8. Concomitant administration of repaglinide with clopidogrel increased the systemic exposure to repaglinide (AUC0-∞) by 5.1-fold following the loading dose (300 mg) and by 3.9-fold on day 3 of the maintenance dose (75 mg) of clopidogrel [see Drug Interactions (7.5)].12.5 Pharmacogenomics
CYP2C19 is involved in the formation of both the active metabolite and the 2-oxo-clopidogrel intermediate metabolite. Clopidogrel active metabolite pharmacokinetics and antiplatelet effects, as measured by ex vivo platelet aggregation assays, differ according to CYP2C19 genotype. Patients who are homozygous for nonfunctional alleles of the CYP2C19 gene are termed "CYP2C19 poor metabolizers". Approximately 2% of White and 4% of Black patients are poor metabolizers; the prevalence of poor metabolism is higher in Asian patients (e.g., 14% of Chinese). Tests are available to identify patients who are CYP2C19 poor metabolizers.
A crossover study in 40 healthy subjects, 10 each in the four CYP2C19 metabolizer groups, evaluated pharmacokinetic and antiplatelet responses using 300 mg followed by 75 mg per day and 600 mg followed by 150 mg per day, each for a total of 5 days. Decreased active metabolite exposure and diminished inhibition of platelet aggregation were observed in the poor metabolizers as compared to the other groups.
Table 3: Active Metabolite Pharmacokinetics and Antiplatelet Responses by CYP2C19 Metabolizer Status
Dose
Poor (n=10)
Intermediate*(n=10)
Normal(n=10)
Ultrarapid†(n=10)
Cmax (ng/mL)
300 mg (24 h)
600 mg (24 h)
75 mg (Day 5)
150 mg (Day 5)11 (4)
17 (6)
4 (1)
7 (2)23 (11)
39 (23)
12 (5)
18 (7)32 (21)
44 (27)
13 (7)
19 (5)24 (10)
36 (13)
12 (6)
16 (9)IPA (%)††
300 mg (24 h)
600 mg (24 h)
75 mg (Day 5)
150 mg (Day 5)24 (26)
32 (25)
37 (23)
61 (14)37 (21)
56 (22)
60 (18)
74 (14)39 (28)
49 (23)
58 (19)
73 (9)40 (21)
51 (28)
56 (13)
68 (18)VASP-PRI (%) †††
300 mg (24 h)
600 mg (24 h)
75 mg (Day 5)
150 mg (Day 5)91 (12)
85 (14)
83 (13)
61 (18)78 (12)
56 (26)
50 (16)
29 (11)68 (16)
48 (20)
39 (14)
24 (10)73 (12)
51 (20)
40 (9)
20 (10)Values are mean (SD)*
Intermediate metabolizers have one but not two nonfunctional alleles
† Ultrarapid metabolizers have at least one gain-of-function allele
†† Inhibition of platelet aggregation with 5mcM ADP; larger value indicates greater platelet inhibition
††† Vasodilator-stimulated phosphoprotein – platelet reactivity index; smaller value indicates greater platelet inhibition -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of tumorigenicity when clopidogrel was administered for 78 weeks to mice and 104 weeks to rats at dosages up to 77 mg/kg per day, which afforded plasma exposures >25 times that in humans at the recommended daily dose of 75 mg.
Clopidogrel was not genotoxic in four in vitro tests (Ames test, DNA-repair test in rat hepatocytes, gene mutation assay in Chinese hamster fibroblasts, and metaphase chromosome analysis of human lymphocytes) and in one in vivo test (micronucleus test by oral route in mice).
Clopidogrel was found to have no effect on fertility of male and female rats at oral doses up to 400 mg/kg per day (52 times the recommended human dose on a mg/m2 basis).
-
14 CLINICAL STUDIES
14.1 Acute Coronary Syndrome
CURE
The CURE study included 12,562 patients with ACS without ST-elevation (UA or NSTEMI) and presenting within 24 hours of onset of the most recent episode of chest pain or symptoms consistent with ischemia. Patients were required to have either ECG changes compatible with new ischemia (without ST-elevation) or elevated cardiac enzymes or troponin I or T to at least twice the upper limit of normal.
Patients were randomized to receive clopidogrel (300-mg loading dose followed by 75 mg once daily) or placebo, and were treated for up to one year. Patients also received aspirin (75 to 325 mg once daily) and other standard therapies such as heparin. The use of GPIIb/IIIa inhibitors was not permitted for three days prior to randomization.
The patient population was largely White (82%) and included 38% women, and 52% age ≥ 65 years of age. Only about 20% of patients underwent revascularization during the initial hospitalization and few underwent emergent or urgent revascularization.
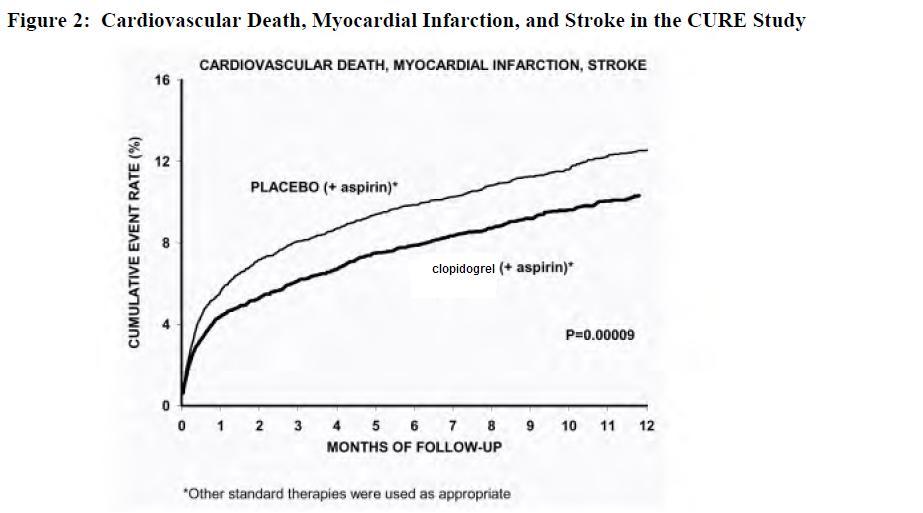
The number of patients experiencing the primary outcome (CV death, MI, or stroke) was 582 (9.3%) in the clopidogrel-treated group and 719 (11.4%) in the placebo-treated group, a 20% relative risk reduction (95% CI of 10% to 28%; p < 0.001) for the clopidogrel-treated group (see Table 4).
Table 4: Outcome Events in the CURE Primary Analysis Outcome Clopidogrel
(+ aspirin)*
(n=6259)Placebo
(+ aspirin)*
(n=6303)Relative Risk
Reduction (%)
(95% CI)Primary outcome
(Cardiovascular death, MI, stroke)582 (9.3%)
719 (11.4%)
20%
(10.3, 27.9)
p < 0.001All Individual Outcome Events:†
CV death
318 (5.1%)
345 (5.5%)
7%
(-7.7, 20.6)MI
324 (5.2%)
419 (6.6%)
23%
(11.0, 33.4)Stroke
75 (1.2%)
87 (1.4%)
14%
(-17.7, 36.6)Most of the benefit of clopidogrel occurred in the first two months, but the difference from placebo was maintained throughout the course of the trial (up to 12 months) (see Figure 2).
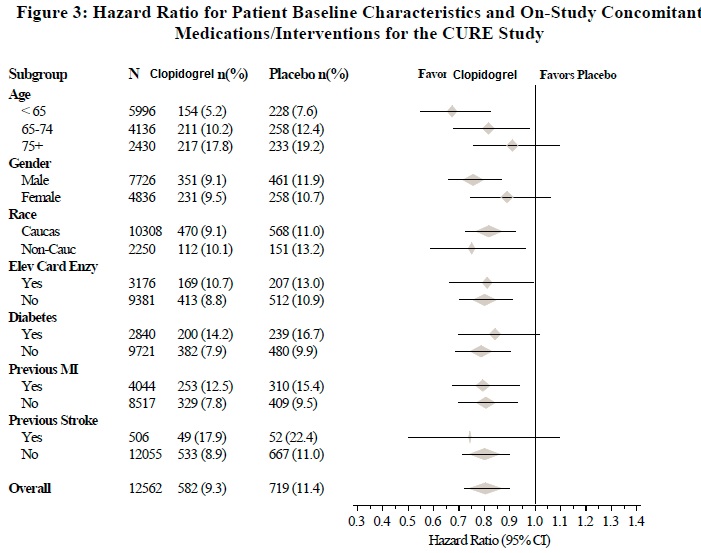
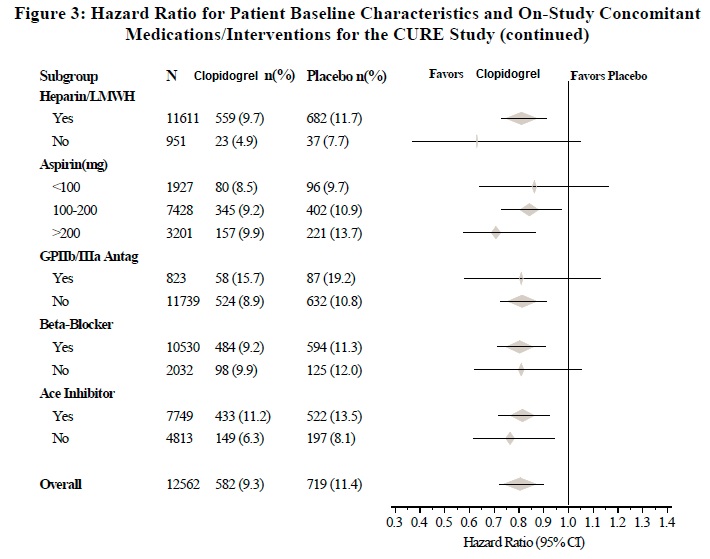
The effect of clopidogrel did not differ significantly in various subgroups, as shown in Figure 3. The benefits associated with clopidogrel were independent of the use of other acute and long-term cardiovascular therapies, including heparin/LMWH, intravenous glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors, lipid-lowering drugs, beta-blockers, and ACE-inhibitors. The efficacy of clopidogrel was observed independently of the dose of aspirin (75 to 325 mg once daily). The use of oral anticoagulants, non-study antiplatelet drugs, and chronic NSAIDs was not allowed in CURE.
The use of clopidogrel in CURE was associated with a decrease in the use of thrombolytic therapy (71 patients [1.1%] in the clopidogrel group, 126 patients [2.0%] in the placebo group; relative risk reduction of 43%), and GPIIb/IIIa inhibitors (369 patients [5.9%] in the clopidogrel group, 454 patients [7.2%] in the placebo group, relative risk reduction of 18%). The use of clopidogrel in CURE did not affect the number of patients treated with CABG or PCI (with or without stenting), (2,253 patients [36.0%] in the clopidogrel group, 2,324 patients [36.9%] in the placebo group; relative risk reduction of 4.0%).
COMMIT
In patients with STEMI, the safety and efficacy of clopidogrel were evaluated in the randomized, placebo-controlled, double-blind study, COMMIT. COMMIT included 45,852 patients presenting within 24 hours of the onset of the symptoms of myocardial infarction with supporting ECG abnormalities (i.e., ST-elevation, ST-depression or left bundle-branch block). Patients were randomized to receive clopidogrel (75 mg once daily) or placebo, in combination with aspirin (162 mg per day), for 28 days or until hospital discharge, whichever came first.
The primary endpoints were death from any cause and the first occurrence of re-infarction, stroke or death.
The patient population was 28% women and 58% age ≥ 60 years (26% age ≥ 70 years). Fifty- five percent (55%) of patients received thrombolytics and only 3% underwent PCI.
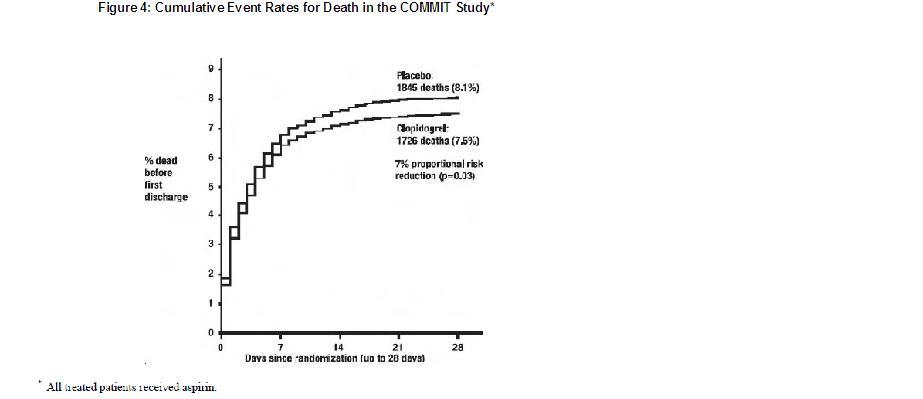
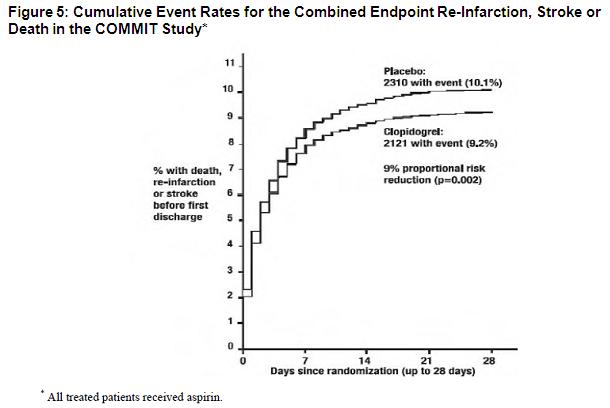
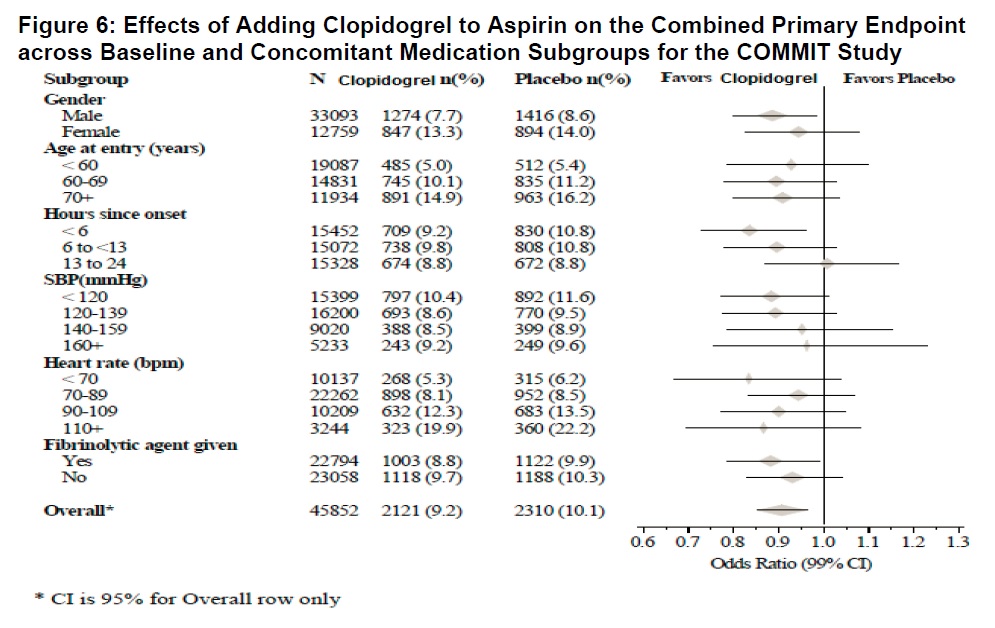
As shown in Table 5 and Figure 4 and Figure 5 below, clopidogrel significantly reduced the relative risk of death from any cause by 7% (p=0.029), and the relative risk of the combination of re-infarction, stroke or death by 9% (p=0.002).
Table 5: Outcome Events in COMMIT
Event
Clopidogrel
(+ aspirin)
(N=22961)Placebo
(+ aspirin)
(N=22891)Odds ratio
(95% CI)p-value
Composite endpoint: Death, MI, or Stroke*
2121 (9.2%)
2310 (10.1%)
0.91 (0.86, 0.97)
0.002
Death
Non-fatal MI**
Non-fatal Stroke**1726 (7.5%)
270 (1.2%)
127 (0.6%)1845 (8.1%)
330 (1.4%)
142 (0.6%)0.93 (0.87, 0.99)
0.81 (0.69, 0.95)
0.89 (0.70, 1.13)0.029
0.011
0.33* 9 patients (2 clopidogrel and 7 placebo) suffered both a non-fatal stroke and a non-fatal MI.
** Non-fatal MI and non-fatal stroke exclude patients who died (of any cause).
The effect of clopidogrel did not differ significantly in various pre-specified subgroups as shown in Figure 6. The effect was also similar in non-prespecified subgroups including those based on infarct location, Killip class or prior MI history. Such subgroup analyses should be interpreted cautiously.
14.2 Recent Myocardial Infarction, Recent Stroke, or Established Peripheral Arterial Disease
CAPRIE
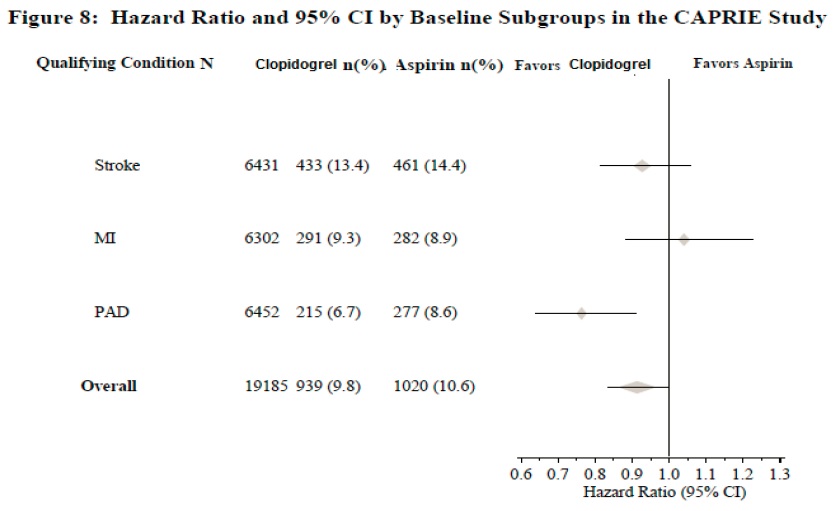
The CAPRIE trial was a 19,185-patient, 304-center, international, randomized, double-blind, parallel-group study comparing clopidogrel (75 mg daily) to aspirin (325 mg daily). To be eligible to enroll, patients had to have: 1) recent history of myocardial infarction (within 35 days); 2) recent histories of ischemic stroke (within 6 months) with at least a week of residual neurological signs; and/or 3) established peripheral arterial disease (PAD). Patients received randomized treatment for an average of 1.6 years (maximum of 3 years).
The trial's primary outcome was the time to first occurrence of new ischemic stroke (fatal or not), new myocardial infarction (fatal or not), or other vascular death. Deaths not easily attributable to nonvascular causes were all classified as vascular.
Table 6: Outcome Events in the CAPRIE Primary Analysis Clopidogrel Aspirin Patients n=9599 n=9586 Ischemic stroke (fatal or not)
438 (4.6%)
461 (4.8%)
MI (fatal or not)
275 (2.9%)
333 (3.5%)
Other vascular death
226 (2.4%)
226 (2.4%)
Total
939 (9.8%)
1020 (10.6%)
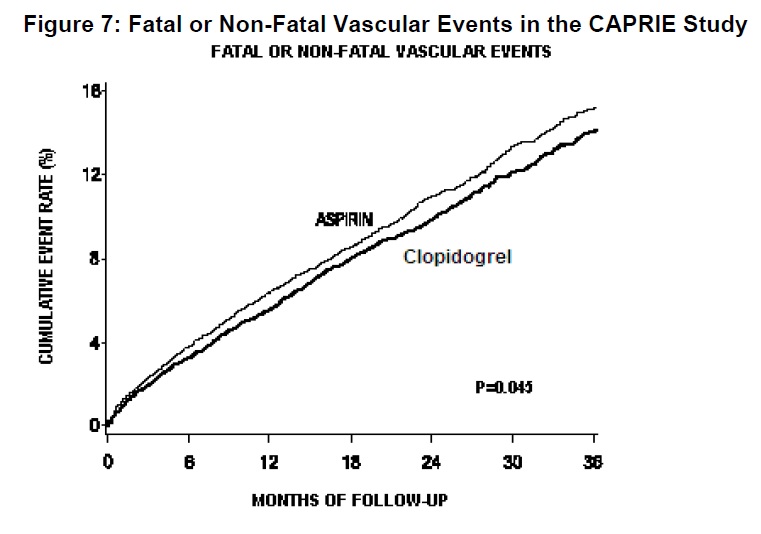
As shown in Table 6, clopidogrel was associated with a lower incidence of outcome events, primarily MI. The overall relative risk reduction (9.8% vs. 10.6%) was 8.7%, p=0.045. Similar results were obtained when all-cause mortality and all-cause strokes were counted instead of vascular mortality and ischemic strokes (risk reduction 6.9%). In patients who survived an on-study stroke or myocardial infarction, the incidence of subsequent events was lower in the clopidogrel group.
The curves showing the overall event rate are shown in Figure 8. The event curves separated early and continued to diverge over the 3-year follow-up period.
The statistical significance favoring clopidogrel over aspirin was marginal (p=0.045). However, because aspirin is itself effective in reducing cardiovascular events in patients with recent myocardial infarction or stroke, the effect of clopidogrel is substantial.
The CAPRIE trial enrolled a population that had recent MI, recent stroke, or PAD. The efficacy of clopidogrel relative to aspirin was heterogeneous across these subgroups (p=0.043) (see Figure 8). Nonetheless this difference may be a chance occurrence because the CAPRIE trial was not designed to evaluate the relative benefit of clopidogrel over aspirin in the individual patient subgroups. The benefit was most apparent in patients who were enrolled because of peripheral arterial disease and less apparent in stroke patients. In patients who were enrolled in the trial on the sole basis of a recent myocardial infarction, clopidogrel was not numerically superior to aspirin.
14.3 No Demonstrated Benefit of Clopidogrel plus Aspirin in Patients with Multiple Risk Factors or Established Vascular Disease
CHARISMA
The CHARISMA trial was a 15,603 subject, randomized, double-blind, parallel group study comparing clopidogrel (75 mg daily) to placebo for prevention of ischemic events in patients with vascular disease or multiple risk factors for atherosclerosis. All subjects were treated with aspirin 75 to 162 mg daily. The mean duration of treatment was 23 months. The study failed to demonstrate a reduction in the occurrence of the primary endpoint, a composite of CV death, MI, or stroke. A total of 534 (6.9%) patients in the clopidogrel group versus 573 (7.4%) patients in the placebo group experienced a primary outcome event (p=0.22). Bleeding of all severities was more common in the subjects randomized to clopidogrel.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Clopidogrel tablets, USP 75 mg are pink, round, biconvex, film coated tablets, engraved "APO" on one side and "CL" over "75" on the other side. They are supplied as follows:
Bottles of 30 NDC 60429-301-30
Bottles of 90 NDC 60429-301-90
Bottles of 1,000 NDC 60429-301-01
Bottles of 1,000 NDC 60429-301-10
Blisters of 100 tablets (10 x 10 Unit Dose) NDC 60429-301-77
-
17 PATIENT COUNSELING INFORMATION
Advise patients to read FDA approved patient labeling (Medication Guide).
Discontinuation
Advise patients not to discontinue clopidogrel without first discussing it with the health care provider who prescribed it [see Warnings and Precautions (5.3)].Bleeding
Advise patients that they:- 1.
- will bruise and bleed more easily
- 2.
- will take longer than usual to stop bleeding
- 3.
- must report any unanticipated, prolonged, or excessive bleeding, or blood in their stool or urine [see Warnings and Precautions (5.2)].
Thrombotic Thrombocytopenic Purpura
Instruct patients to get prompt medical attention if they experience symptoms of TTP that cannot otherwise be explained [see Warnings and Precautions (5.4)].Invasive Procedures
Advise patients to inform physicians and dentists that they are taking clopidogrel before any surgery or dental procedure [see Warnings and Precautions (5.2, 5.3)].Proton Pump Inhibitors
Advise patients not to take omeprazole or esomeprazole while taking clopidogrel. Dexlansoprazole, lansoprazole and pantoprazole had less pronounced effects on the antiplatelet activity of clopidogrel than did omeprazole or esomeprazole [see Drug Interactions (7.1)].GSMS, INC.
CLOPIDOGREL TABLETS, USP
75 mgManufactured by
Manufactured for
Marketed/ Packaged by:
Apotex Inc.
Apotex Corp.
GSMS, Inc.
Toronto, Ontario
Weston, Florida
Camarillo, CA
Canada M9L 1T9
33326
93010
Revised: November 2016
Rev. 21
-
Medication Guide
Clopidogrel Bisulfate Tablets, USP 75 mg and 300 mg
(kloe pid’ oh grel bye sul’ fate)
Read this Medication Guide before you start taking clopidogrel tablets and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about clopidogrel tablets?
1. Clopidogrel tablets may not work as well in people who:
- 1.
-
have certain genetic factors that affect how the body breaks down clopidogrel.
Your doctor may do genetic tests to make sure clopidogrel is right for you. - 2.
-
take certain medicines, especially omeprazole (Prilosec®) or esomeprazole (Nexium®).
Your doctor may change the medicine you take for stomach acid problems while you take clopidogrel tablets.
2. Clopidogrel tablets can cause bleeding which can be serious and can sometimes lead to death. Clopidogrel is a blood thinner medicine that lowers the chance of blood clots forming in your body. While you take clopidogrel tablets:
- 1.
- you may bruise and bleed more easily
- 2.
- you are more likely to have nose bleeds
- 3.
- it will take longer for any bleeding to stop
Call your doctor right away if you have any of these signs or symptoms of bleeding:
- •
- unexpected bleeding or bleeding that lasts a long time
- •
- blood in your urine (pink, red or brown urine)
- •
- red or black stools (looks like tar)
- •
- bruises that happen without a known cause or get larger
- •
- cough up blood or blood clots
- •
- vomit blood or your vomit looks like coffee grounds
Do not stop taking clopidogrel tablets without talking to the doctor who prescribes it for you. People who stop taking clopidogrel tablets too soon have a higher risk of having a heart attack or dying. If you must stop clopidogrel tablets because of bleeding, your risk of a heart attack may be higher.
What are clopidogrel tablets?
Clopidogrel bisulfate tablets are a prescription medicine used to treat people who have any of the following:
- •
- chest pain due to heart problems
- •
- poor circulation in their legs (peripheral arterial disease)
- •
- a heart attack
- •
- a stroke
Clopidogrel is used alone or with aspirin to lower your chance of having another serious problem with your heart or blood vessels such as heart attack, stroke, or blood clot that can lead to death.
Platelets are blood cells that help your blood clot normally. Clopidogrel helps to prevent platelets from sticking together and forming a clot that can block an artery.
It is not known if clopidogrel tablets are safe and effective in children.
Who should not take clopidogrel tablets?
Do not take clopidogrel tablets if you:
- •
- currently have a condition that causes bleeding, such as a stomach ulcer
- •
- are allergic to clopidogrel or other ingredients in clopidogrel tablets. See the end of this leaflet for a complete list of ingredients in clopidogrel tablets.
What should I tell my doctor before taking clopidogrel tablets?
Before you take clopidogrel tablets, tell your doctor if you:
- •
- have a history of bowel (gastrointestinal) or stomach ulcers
- •
- have a history of bleeding problems
- •
- plan to have surgery or a dental procedure. See "How should I take clopidogrel tablets?"
- •
- are pregnant or plan to become pregnant. It is not known if clopidogrel will harm your unborn baby
- •
- are breastfeeding or plan to breastfeed. It is not known if clopidogrel passes into your breast milk. You and your doctor should decide if you will take clopidogrel tablets or breastfeed. You should not do both without talking to your doctor
- •
- have had an allergy or reaction to any medicine used to treat your disease.
Tell all of your doctors and your dentist that you are taking clopidogrel tablets. They should talk to the doctor who prescribed clopidogrel tablets for you before you have any surgery or invasive procedure.
Tell your doctor about all the medicines you take, including prescription, non-prescription medicines, vitamins and herbal supplements.
Clopidogrel tablets may affect the way other medicines work, and other medicines may affect how clopidogrel tablets work. See "What is the most important information I should know about clopidogrel tablets?"
Clopidogrel may increase blood levels of other medicines such as repaglinide (Prandin®).
Taking clopidogrel tablets with certain other medicines may increase your risk of bleeding. Especially tell your doctor if you take:
- •
- aspirin, especially if you have had a stroke. Always talk to your doctor about whether you should take aspirin along with clopidogrel to treat your condition.
- •
- Non-steroidal anti-inflammatory drugs (NSAIDs). Ask your doctor or pharmacist for a list of NSAID medicines if you are not sure.
- •
- warfarin (Coumadin®, Jantoven®)
- •
- selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs). Ask your doctor or pharmacist for a list of SSRI or SNRI medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor or pharmacist when you get a new medicine.
How should I take clopidogrel tablets?
- •
- Take clopidogrel tablets exactly as your doctor tells you.
- •
- Do not change your dose or stop taking clopidogrel tablets without talking to your doctor first. Stopping clopidogrel tablets may increase your risk of heart attack or stroke.
- •
- Take clopidogrel tablets with aspirin as instructed by your doctor.
- •
- If you miss a dose, take clopidogrel tablets as soon as you remember. If it is almost time for your next dose, skip the missed dose. Take the next dose at your regular time. Do not take 2 doses of clopidogrel tablets at the same time unless your doctor tells you to.
- •
- If you take too much clopidogrel, call your doctor or go to the nearest emergency room right away.
- •
- Talk with your doctor about stopping your clopidogrel tablets before you have surgery. Your doctor may tell you to stop taking clopidogrel tablets at least 5 days before you have surgery to avoid excessive bleeding during surgery.
What are the possible side effects of clopidogrel tablets?
Clopidogrel tablets can cause serious side effects including:
- •
- See "What is the most important information I should know about clopidogrel tablets?"
- •
-
A blood clotting problem called Thrombotic Thrombocytopenic Purpura (TTP). TTP can happen with clopidogrel, sometimes after a short time (less than 2 weeks). TTP is a blood clotting problem where blood clots form in blood vessels; and can happen anywhere in the body. TTP needs to be treated in a hospital right away, because it may cause death. Get medical help right away if you have any of these symptoms and they can not be explained by another medical condition:
- •
- purplish spots (called purpura) on the skin or in the mouth (mucous membranes) due to bleeding under the skin
- •
- your skin or the whites of your eyes are yellow (jaundice)
- •
- you feel tired or weak
- •
- your skin looks very pale
- •
- fever
- •
- fast heart rate or feeling short of breath
- •
- headache
- •
- speech changes
- •
- confusion
- •
- coma
- •
- stroke
- •
- seizure
- •
- low amount of urine, or urine that is pink or has blood in it
- •
- stomach area (abdominal) pain
- •
- nausea, vomiting, or diarrhea
- •
- vision changes
Tell your doctor if you have any side effect that bothers you or that does not go away.
Tell your doctor if you develop an allergic reaction including skin reactions while taking clopidogrel tablets.
These are not all the possible side effects of clopidogrel tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store clopidogrel tablets?
- •
- Store clopidogrel tablets at 59°F to 86°F (15°C to 30°C). Protect from moisture.
Keep clopidogrel and all medicines out of the reach of children.
General information about clopidogrel tablets
Medicines are sometimes used for purposes other than those listed in a Medication Guide. Do not take clopidogrel tablets for a condition for which it was not prescribed. Do not give clopidogrel tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about clopidogrel tablets. If you would like more information, talk to your doctor. Ask your doctor or pharmacist for information about clopidogrel that was written for healthcare professionals.
For more information, go to www.apotex.com or call 1-800-706-5575.
What are the ingredients in clopidogrel tablets?
Active Ingredient: clopidogrel bisulfate
Inactive Ingredients:
Tablet: anhydrous lactose, colloidal silicon dioxide, crospovidone, methylcellulose and zinc stearate
Film coating: hydroxypropyl cellulose, hypromellose, polyethylene glycol, red ferric oxide and titanium dioxide.
All registered trademarks in this document are the property of their respective owners.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
GSMS, Inc.
CLOPIDOGREL TABLETS, USP 75 mg
Manufactured by
Manufactured for
Marketed/ Packaged by
Apotex Inc.
Apotex Corp.
GSMS, Inc.
Toronto, Ontario
Weston, Florida
Camarillo, CA
Canada M9L 1T9
33326
93010
Revised: November 2016
Rev. 21
Coumadin® is a registered trademark of Bristol-Myers Squibb Pharma Company.
Prilosec® is a registered trademark of AstraZeneca.
Jantoven® is a registered trademark of USL Pharma. -
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
Count Strength mg 30 43353-269-30 90 43353-269-60 Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20171011DKJ - PRINCIPAL DISPLAY PANEL - 75mg
-
INGREDIENTS AND APPEARANCE
CLOPIDOGREL BISULFATE
clopidogrel bisulfate tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43353-269(NDC:60429-301) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLOPIDOGREL BISULFATE (UNII: 08I79HTP27) (CLOPIDOGREL - UNII:A74586SNO7) CLOPIDOGREL 75 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) METHYLCELLULOSE (15 MPA.S) (UNII: NPU9M2E6L8) ZINC STEARATE (UNII: H92E6QA4FV) FERRIC OXIDE RED (UNII: 1K09F3G675) HYDROXYPROPYL CELLULOSE (1200000 MW) (UNII: RFW2ET671P) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score no score Shape ROUND Size 9mm Flavor Imprint Code APO;CL;75 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43353-269-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2017 2 NDC:43353-269-60 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/18/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076274 05/17/2012 Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, LLC 128385585 REPACK(43353-269)