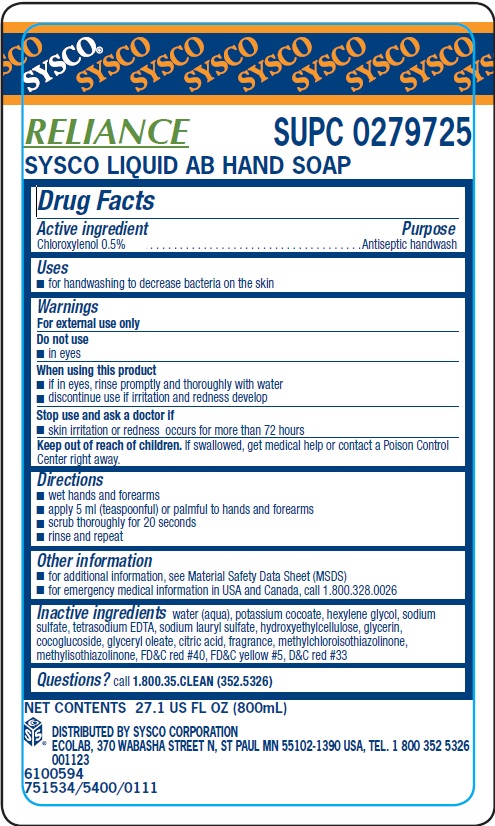
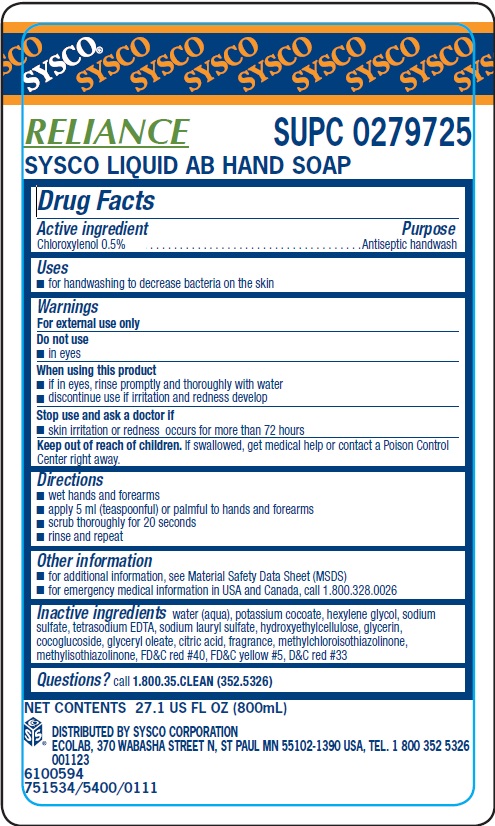
Label: SYSCO- chloroxylenol solution
- NDC Code(s): 47593-479-25
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
INACTIVE INGREDIENT
Inactive ingredients water (aqua), potassium cocoate, hexylene glycol, sodium sulfate, tetrasodium EDTA, sodium lauryl sulfate, hydroxyehtylcellulose, glycerin, cocoglucoside, glyceryl oleate, citric acid, fragrance, methylchloroisothiazolinone, methylisothiazolinone, FDC red 40, FDC yellow 5, DC red 33
- QUESTIONS
- Principal display panel and representative label
-
INGREDIENTS AND APPEARANCE
SYSCO
chloroxylenol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-479 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 0.5 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM COCOATE (UNII: F8U72V8ZXP) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM SULFATE (UNII: 0YPR65R21J) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) GLYCERIN (UNII: PDC6A3C0OX) COCO GLUCOSIDE (UNII: ICS790225B) GLYCERYL OLEATE (UNII: 4PC054V79P) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-479-25 800 mL in 1 POUCH; Type 0: Not a Combination Product 03/31/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 03/31/2011 Labeler - Ecolab Inc. (006154611)