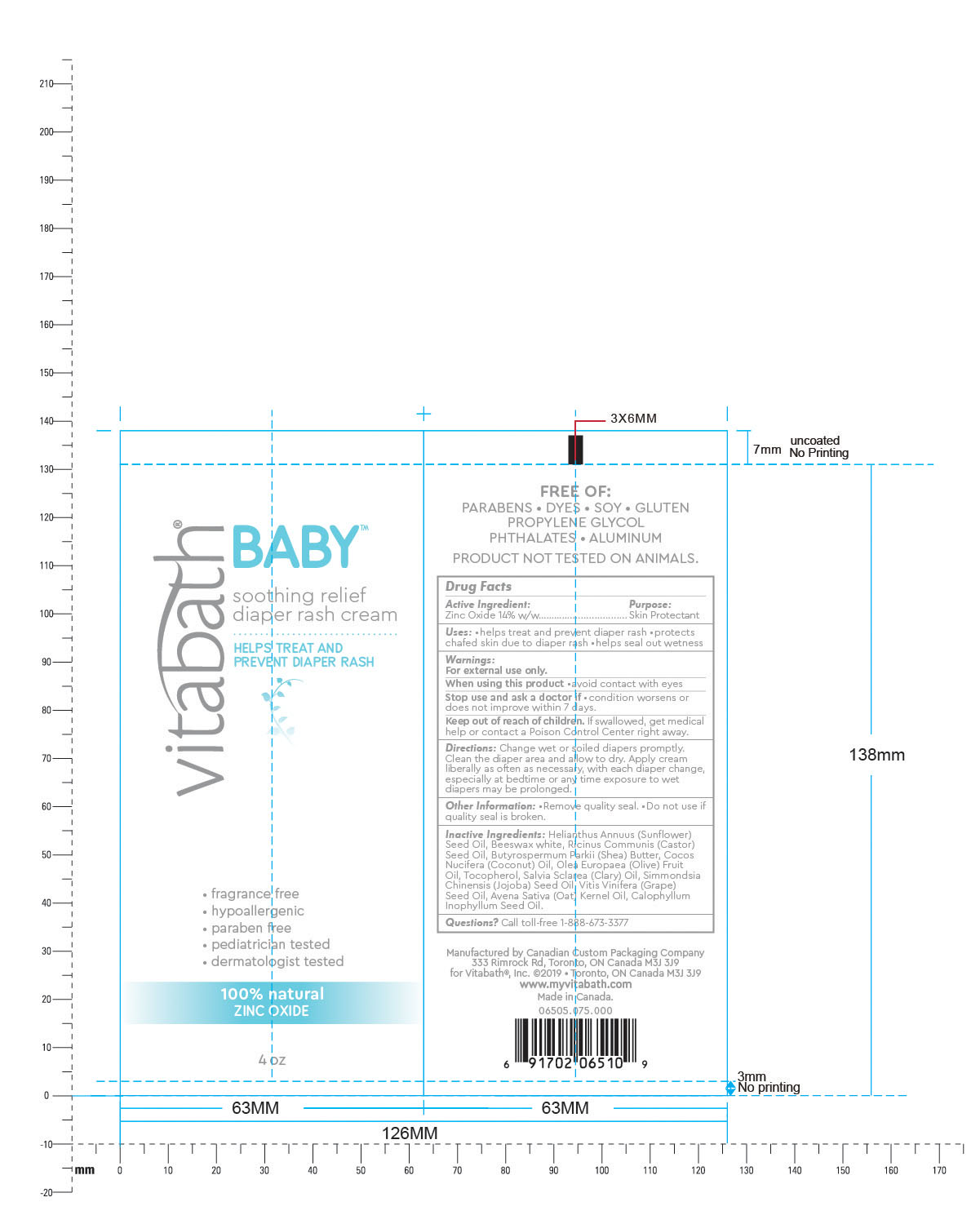
Label: VITABATH BABY SOOTHING RELIEF DIAPER RASH- zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 73172-004-01 - Packager: Vitabath Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 13, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive Ingredients: Helianthus Annuus (Sunflower)
Seed Oil, Beeswax white, Ricinus Communis (Castor)
Seed Oil, Butyrospermum Parkii (Shea) Butter, Cocos
Nucifera (Coconut) Oil, Olea Europaea (Olive) Fruit
Oil, Tocopherol, Salvia Sclarea (Clary) Oil, Simmondsia
Chinensis (Jojoba) Seed Oil, Vitis Vinifera (Grape)
Seed Oil, Avena Sativa (Oat) Kernel Oil, Calophyllum
Inophyllum Seed Oil. - QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITABATH BABY SOOTHING RELIEF DIAPER RASH
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73172-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14 g in 100 g Inactive Ingredients Ingredient Name Strength TAMANU OIL (UNII: JT3LVK84A1) GRAPE SEED OIL (UNII: 930MLC8XGG) SUNFLOWER OIL (UNII: 3W1JG795YI) WHITE WAX (UNII: 7G1J5DA97F) CASTOR OIL (UNII: D5340Y2I9G) SHEA BUTTER (UNII: K49155WL9Y) OAT KERNEL OIL (UNII: 3UVP41R77R) COCONUT OIL (UNII: Q9L0O73W7L) OLIVE OIL (UNII: 6UYK2W1W1E) TOCOPHEROL (UNII: R0ZB2556P8) CLARY SAGE OIL (UNII: 87L0D4U3M0) JOJOBA OIL (UNII: 724GKU717M) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73172-004-01 113 g in 1 TUBE; Type 0: Not a Combination Product 06/13/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 06/13/2019 Labeler - Vitabath Inc. (203461637) Establishment Name Address ID/FEI Business Operations Canadian Custom Packaging Comapany 207062514 manufacture(73172-004)