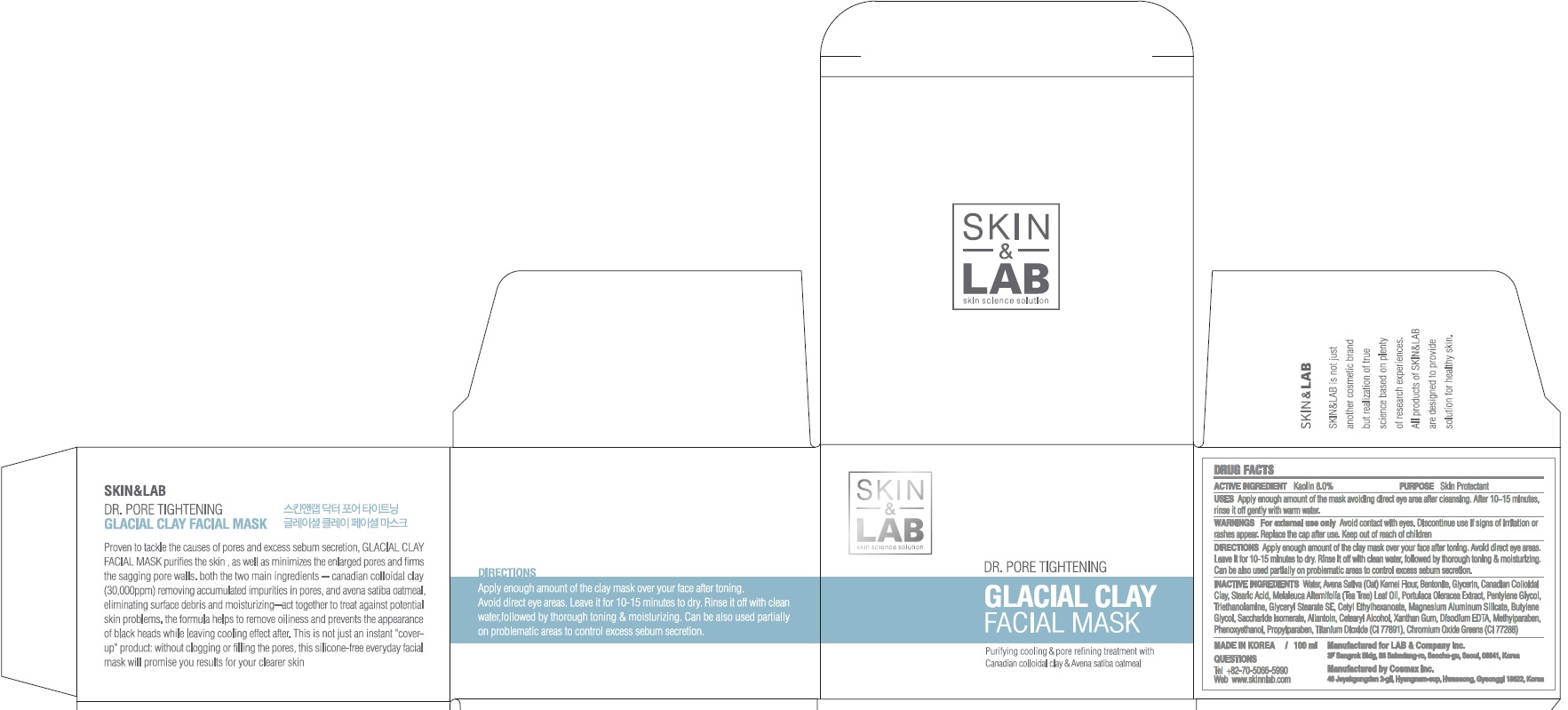
Label: DR PORE TIGHTENING GLACIAL CLAY FACIAL MASK- kaolin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 69457-050-01, 69457-050-02 - Packager: LAB & Company Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 7, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients: Water, Avena Sativa (Oat) Kernel Flour, Bentonite, Glycerin, Canadian Colloidal Clay, Stearic Acid, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Portulaca Oleracea Extract, Pentylene Glycol, Triethanolamine, Glyceryl Stearate SE, Cetyl Ethylhexanoate, Magnesium Aluminum Silicate, Butylene Glycol, Saccharide Isomerate, Allantoin, Cetearyl Alcohol, Xanthan Gum, Disodium EDTA, Methylparaben, Phenoxyethanol, Propylparaben, Titanium Dioxide (CI 77891), Chromium Oxide Greens (CI 77288)
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- QUESTIONS
- Uses
- Directions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR PORE TIGHTENING GLACIAL CLAY FACIAL MASK
kaolin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69457-050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Kaolin (UNII: 24H4NWX5CO) (KAOLIN - UNII:24H4NWX5CO) Kaolin 8.0 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69457-050-02 1 in 1 CARTON 11/01/2017 1 NDC:69457-050-01 100 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 11/01/2017 Labeler - LAB & Company Inc. (688284779) Registrant - LAB & Company Inc. (688284779) Establishment Name Address ID/FEI Business Operations Cosmax, Inc. 689049693 manufacture(69457-050)