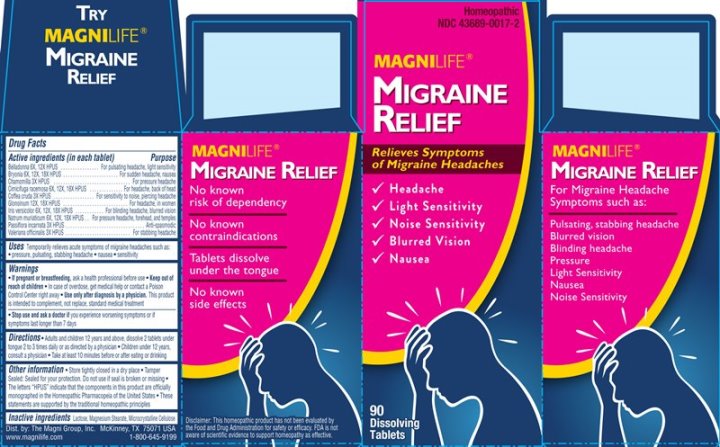
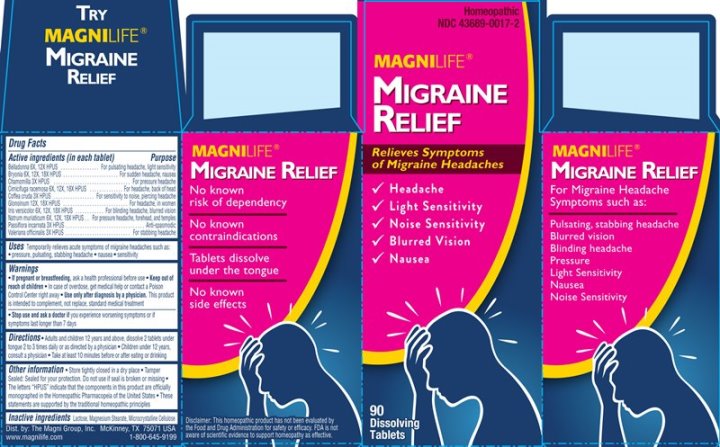
Label: MIGRAINE RELIEF (belladonna, bryonia- alba, chamomilla, cimicifuga racemosa, coffea cruda, glonoinum, iris versicolor, natrum muriaticum, passiflora incarnata, valeriana officinalis tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 43689-0017-1, 43689-0017-2 - Packager: The Magni Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
- USES:
-
WARNINGS:
If pregnant or breastfeeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Use only after diagnosis by a physician. This product is intended to complement, not replace, proper medical attention.
Stop use and ask a doctor if you experience worsening symptoms or if symptoms last longer than 7 days.
Other Information
Store tightly closed in dry place.
Tamper Sealed: Sealed for your protection. Do not use if seal is broken or missing.
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic
Pharmacopeia of the United States.
These statements are supported by the traditional homeopathic principles.
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- USES:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
MIGRAINE RELIEF
belladonna, bryonia (alba), chamomilla, cimicifuga racemosa, coffea cruda, glonoinum, iris versicolor, natrum muriaticum, passiflora incarnata, valeriana officinalis tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43689-0017 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 6 [hp_X] BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] MATRICARIA CHAMOMILLA WHOLE (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA WHOLE 3 [hp_X] BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 6 [hp_X] ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 3 [hp_X] NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 12 [hp_X] IRIS VERSICOLOR ROOT (UNII: X43D4L3DQC) (IRIS VERSICOLOR ROOT - UNII:X43D4L3DQC) IRIS VERSICOLOR ROOT 6 [hp_X] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6 [hp_X] PASSIFLORA INCARNATA FLOWERING TOP (UNII: CLF5YFS11O) (PASSIFLORA INCARNATA FLOWERING TOP - UNII:CLF5YFS11O) PASSIFLORA INCARNATA FLOWERING TOP 3 [hp_X] VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 3 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color white Score no score Shape ROUND (convex) Size 6mm Flavor Imprint Code ML Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43689-0017-2 1 in 1 CARTON 07/17/2014 1 NDC:43689-0017-1 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/17/2014 Labeler - The Magni Company (113501902) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43689-0017) , api manufacture(43689-0017) , label(43689-0017) , pack(43689-0017)