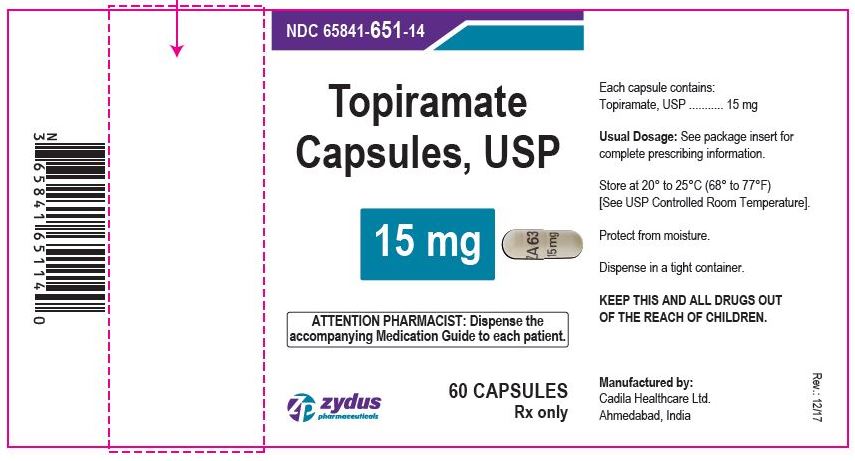
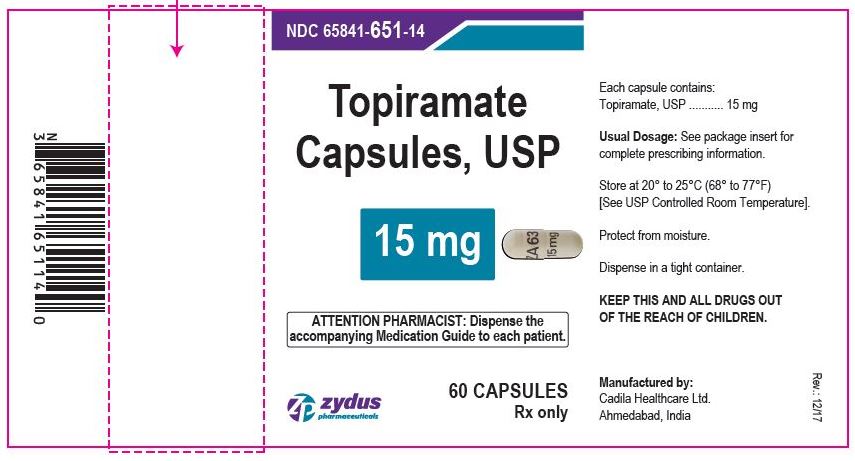
Label: TOPIRAMATE capsule, coated pellets
-
NDC Code(s):
65841-651-01,
65841-651-05,
65841-651-10,
65841-651-14, view more65841-651-16, 65841-652-01, 65841-652-05, 65841-652-10, 65841-652-14, 65841-652-16, 65841-652-17
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOPIRAMATE
topiramate capsule, coated pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-651 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOPIRAMATE (UNII: 0H73WJJ391) (TOPIRAMATE - UNII:0H73WJJ391) TOPIRAMATE 15 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) CELLULOSE ACETATE (UNII: 3J2P07GVB6) GELATIN (UNII: 2G86QN327L) POVIDONE (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (WHITE) Score no score Shape CAPSULE (CAPSULE) Size 18mm Flavor Imprint Code ZA63;15mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-651-14 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 2 NDC:65841-651-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 3 NDC:65841-651-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 4 NDC:65841-651-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 5 NDC:65841-651-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078877 10/14/2009 TOPIRAMATE
topiramate capsule, coated pelletsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-652 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOPIRAMATE (UNII: 0H73WJJ391) (TOPIRAMATE - UNII:0H73WJJ391) TOPIRAMATE 25 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) CELLULOSE ACETATE (UNII: 3J2P07GVB6) GELATIN (UNII: 2G86QN327L) POVIDONE (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (WHITE) Score no score Shape CAPSULE (CAPSULE) Size 19mm Flavor Imprint Code ZA64;25mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-652-17 28 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 2 NDC:65841-652-14 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 3 NDC:65841-652-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 4 NDC:65841-652-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 5 NDC:65841-652-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 6 NDC:65841-652-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078877 10/14/2009 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(65841-651, 65841-652) , MANUFACTURE(65841-651, 65841-652)