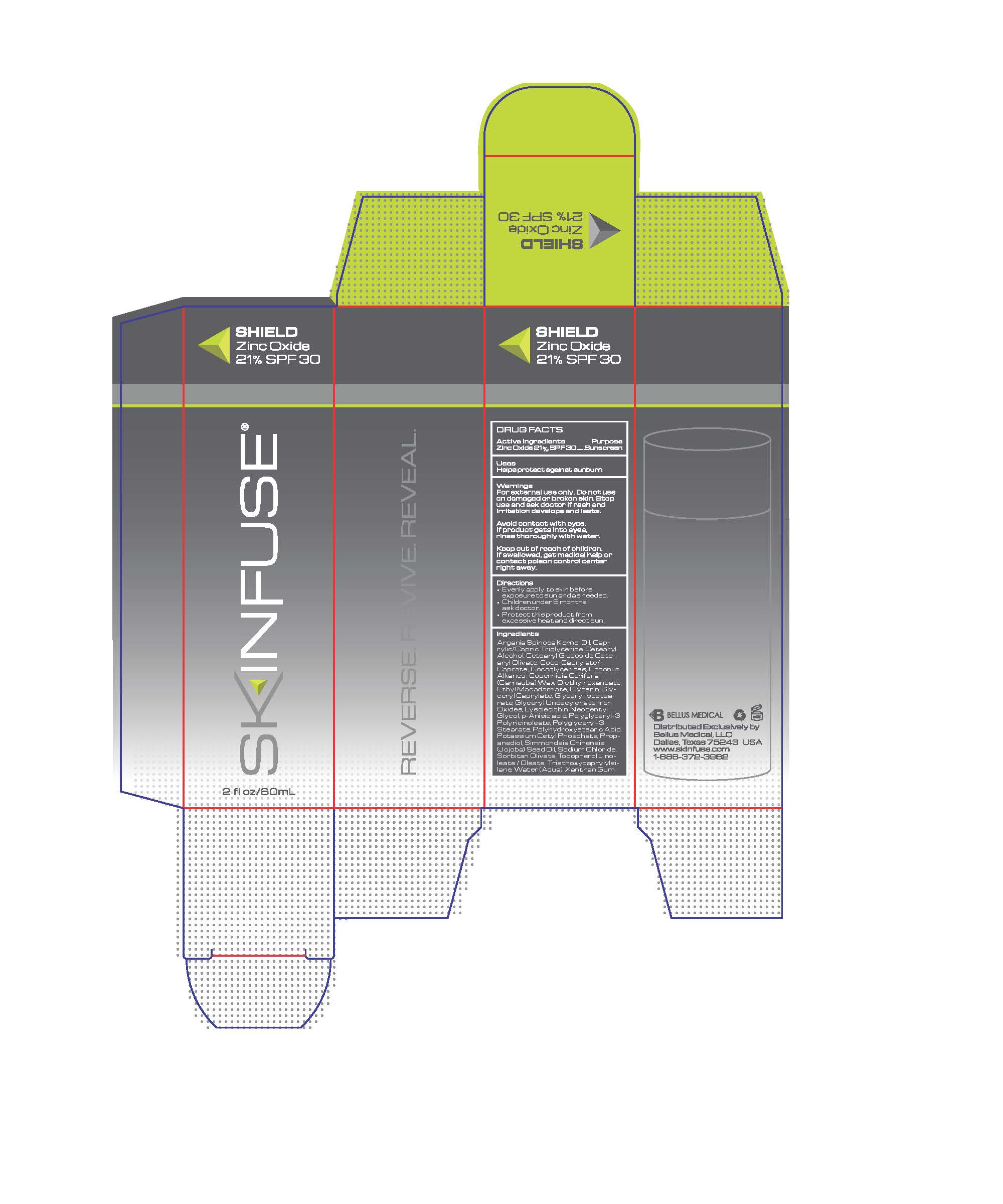
Label: SHIELD- zinc oxide cream
- NDC Code(s): 71888-104-01
- Packager: Bellus Medical, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- INDICATIONS & USAGE
-
INACTIVE INGREDIENT
Argania Spinosa Kernel Oil, Caprylic/
Capric Triglyceride, Cetearyl
Alcohol, Cetearyl Glucoside,Cetearyl
Olivate, Coco-Caprylate/-
Caprate , Cocoglycerides, Coconut
Alkanes , Copernicia Cerifera
(Carnauba) Wax, Diethylhexanoate,
Ethyl Macadamiate, Glycerin, Glyceryl
Caprylate, Glyceryl Isostearate,
Glyceryl Undecylenate, Iron
Oxides, Lysolecithin, Neopentyl
Glycol, p-Anisic acid, Polyglyceryl-3
Polyricinoleate, Polyglyceryl-3
Stearate, Polyhydroxystearic Acid,
Potassium Cetyl Phosphate, Propanediol,
Simmondsia Chinensis
(Jojoba) Seed Oil, Sodium Chloride,
Sorbitan Olivate, Tocopherol Linoleate
/ Oleate, Triethoxycaprylylsilane,
Water (Aqua), Xanthan Gum. - KEEP OUT OF REACH OF CHILDREN
- QUESTIONS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SHIELD
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71888-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 21 g in 100 mL Inactive Ingredients Ingredient Name Strength CETEARYL OLIVATE (UNII: 58B69Q84JO) COCONUT ALKANES (UNII: 1E5KJY107T) PROPANEDIOL (UNII: 5965N8W85T) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) TRICAPRYLIN (UNII: 6P92858988) CARNAUBA WAX (UNII: R12CBM0EIZ) XANTHAN GUM (UNII: TTV12P4NEE) ARGAN OIL (UNII: 4V59G5UW9X) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) COCO-CAPRYLATE (UNII: 4828G836N6) SODIUM CHLORIDE (UNII: 451W47IQ8X) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) WATER (UNII: 059QF0KO0R) ETHYL MACADAMIATE (UNII: ANA2NCS6V1) GLYCERIN (UNII: PDC6A3C0OX) SORBITAN OLIVATE (UNII: MDL271E3GR) JOJOBA OIL (UNII: 724GKU717M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71888-104-01 30 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/01/2015 Labeler - Bellus Medical, LLC (005677967) Registrant - Bellus Medical, LLC (005677967)