Label: VITAMIN B12 3000 MCG- cyanocobalamin injection, solution
- NDC Code(s): 13985-426-04
- Packager: MWI/Vet One
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
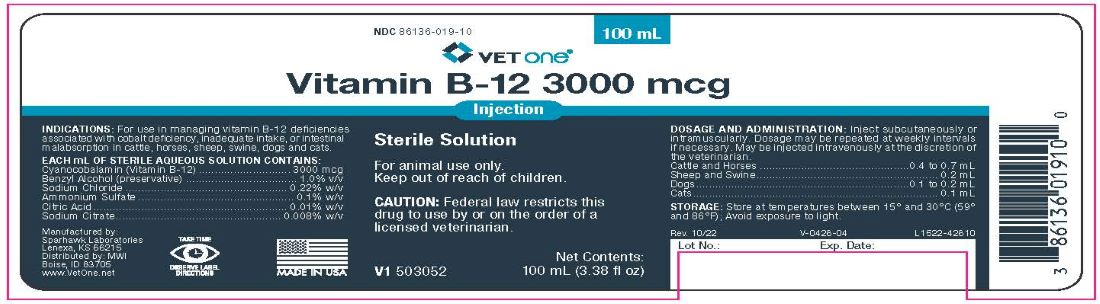
- INDICATIONS & USAGE
- CONTAINS
- STORAGE
-
DOSAGE AND ADMINISTRATION
Inject subcutaneously or intramuscularly. Dosage may be repeated at weekly intervals if necessary. May be injected intravenously at the discretion of the veterinarian.
Cattle and Horses ...............................................0.4 to 0.7 mL
Sheep and Swine......................................................... 0.2 mL
Dogs...................................................................0.1 to 0.2 mL
Cats............................................................................. 0.1 mL
TAKE TIME OBSERVE LABEL DIRECTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN B12 3000 MCG
cyanocobalamin injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:13985-426 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 3000 ug in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-426-04 100 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/02/2011 Labeler - MWI/Vet One (019926120)