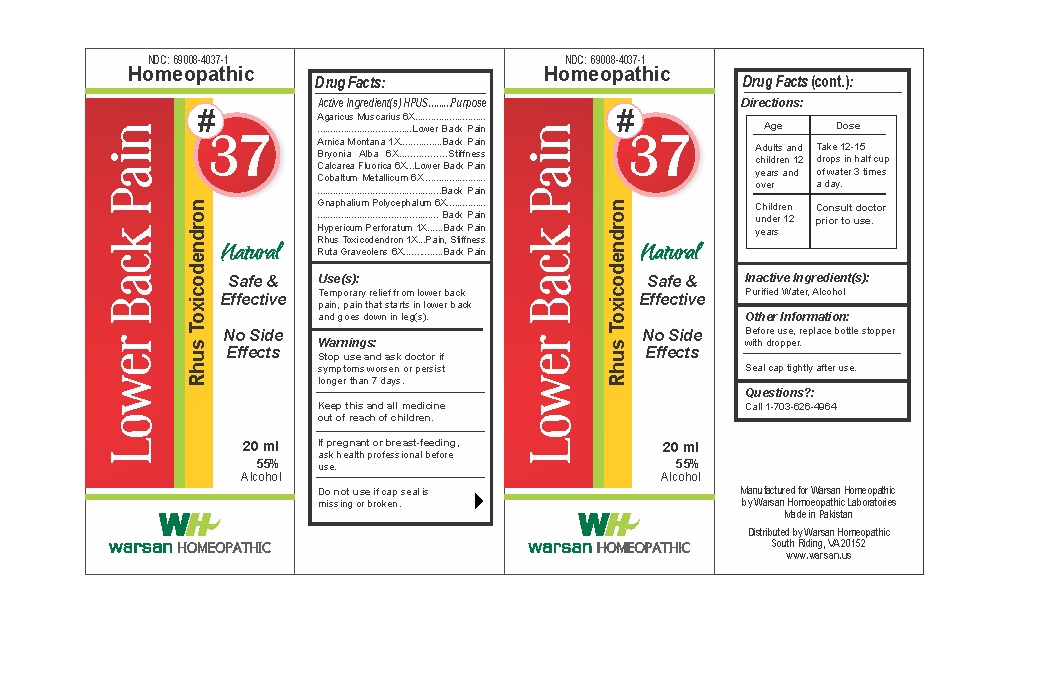
Label: COMBINATION REMEDY 37 - LOW BACK PAIN- arnica montana, bryonia alba, calcarea fluorica, cobaltum metallicum, gnaphalium polycephalum 6x, hypericum perforatum, rhus toxicodendron, ruta graveolens solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 69008-4037-1 - Packager: Warsan Homoeopathic Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 25, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- ACTIVE INGREDIENT
- PURPOSE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COMBINATION REMEDY 37 - LOW BACK PAIN
arnica montana, bryonia alba, calcarea fluorica, cobaltum metallicum, gnaphalium polycephalum 6x, hypericum perforatum, rhus toxicodendron, ruta graveolens solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69008-4037 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 1 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 6 [hp_X] in 1 mL AMANITA MUSCARIA FRUITING BODY (UNII: DIF093I037) (AMANITA MUSCARIA FRUITING BODY - UNII:DIF093I037) AMANITA MUSCARIA FRUITING BODY 6 [hp_X] in 1 mL PSEUDOGNAPHALIUM OBTUSIFOLIUM (UNII: 36XQ854NWW) (PSEUDOGNAPHALIUM OBTUSIFOLIUM - UNII:36XQ854NWW) PSEUDOGNAPHALIUM OBTUSIFOLIUM 6 [hp_X] in 1 mL HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 1 [hp_X] in 1 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 1 [hp_X] in 1 mL COBALT (UNII: 3G0H8C9362) (COBALT - UNII:3G0H8C9362) COBALT 6 [hp_X] in 1 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 1 [hp_X] in 1 mL RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69008-4037-1 20 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 11/16/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/15/2017 Labeler - Warsan Homoeopathic Laboratories (645579772) Registrant - Warsan Homoeopathic Laboratories (645579772) Establishment Name Address ID/FEI Business Operations Warsan Homoeopathic Laboratories 645579772 manufacture(69008-4037) , pack(69008-4037) , label(69008-4037)