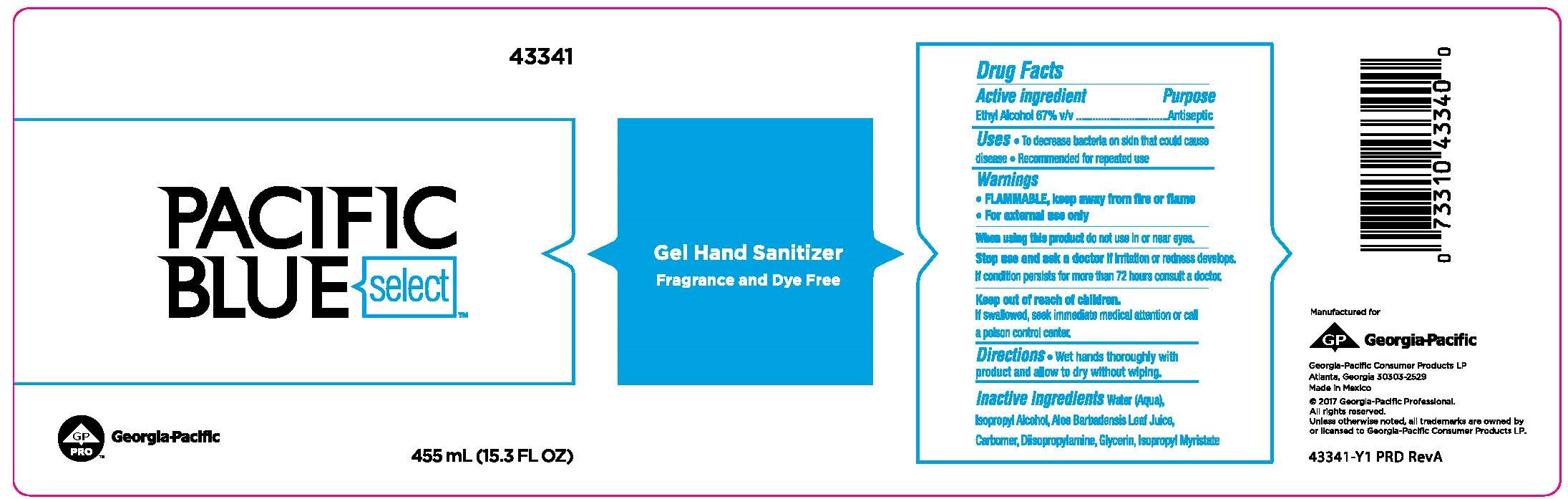
Label: PACIFIC BLUE SELECT GEL HAND SANITIZER FRAGRANCE AND DYE FREE- ethyl alcohol liquid
- NDC Code(s): 54622-308-01
- Packager: Georgia-Pacific Consumer Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 31, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
Pacific Blue Select™
Gel Hand Sanitizer
Fragrance and Dye Free
455mL (15.3 FL OZ)
Georgia-Pacific Consumer Products LP
Atlanta, GA 30303-2529
Made in Mexico
©2017 Georgia-Pacific Professional.
All rights reserved.
Unless otherwise noted, all trademarks are owned by
or licensed to Georgia-Pacific Consumer Products LP.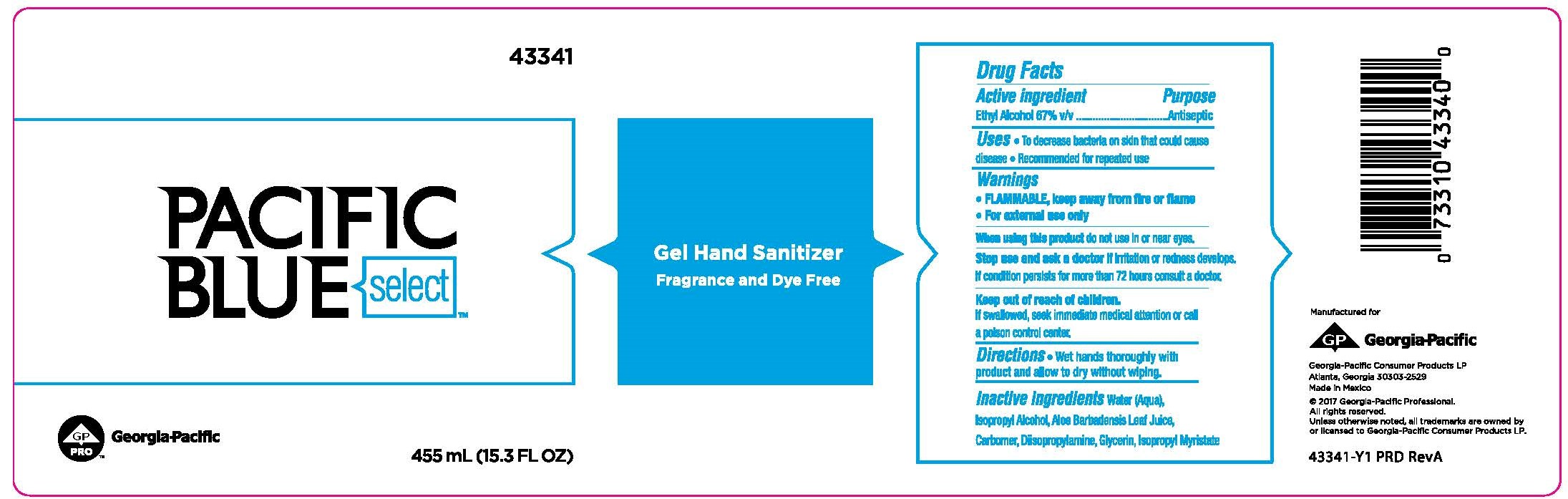
-
INGREDIENTS AND APPEARANCE
PACIFIC BLUE SELECT GEL HAND SANITIZER FRAGRANCE AND DYE FREE
ethyl alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54622-308 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 67 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) ALOE VERA LEAF (UNII: ZY81Z83H0X) SODIUM BENZOATE (UNII: OJ245FE5EU) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM SULFITE (UNII: VTK01UQK3G) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) DIISOPROPYLAMINE (UNII: BR9JLI40NO) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54622-308-01 12 in 1 BOX 10/11/2017 1 455 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 10/11/2017 Labeler - Georgia-Pacific Consumer Products (806142217) Registrant - CYAN Labs S.A. de C.V. (812754130) Establishment Name Address ID/FEI Business Operations CYAN Labs S.A. de C.V. 812754130 manufacture(54622-308) , label(54622-308) , pack(54622-308)