Label: CLINPRO TOOTH CREME- sodium fluoride paste, dentifrice
- NDC Code(s): 48878-3150-1, 48878-3150-2, 48878-3150-5
- Packager: Solventum US OpCo LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
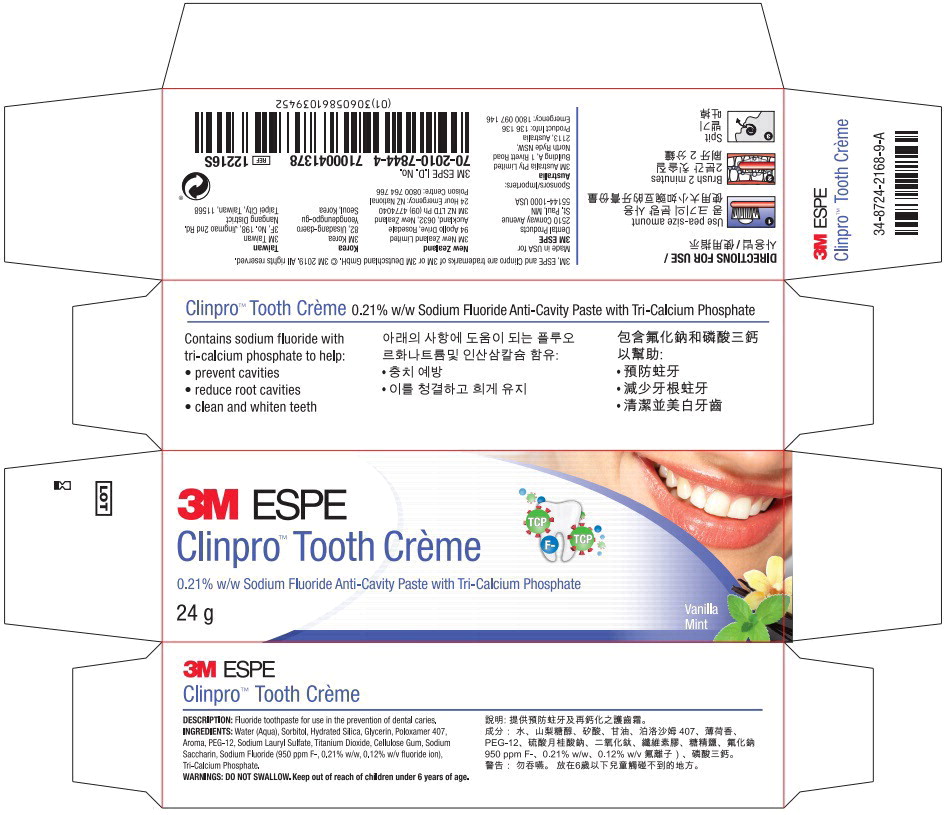
- Principal Display Panel - 24g Carton Label
- Principal Display Panel – 24g Tube Label
- Principal Display Panel - 90 mL Carton Label
- Principal Display Panel - 90 mL Tube Label
- Principal Display Panel - 113g Carton Label
- Principal Display Panel - 113g Tube Label
-
INGREDIENTS AND APPEARANCE
CLINPRO TOOTH CREME
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:48878-3150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sodium fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 0.950 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Sorbitol (UNII: 506T60A25R) Silicon Dioxide (UNII: ETJ7Z6XBU4) Glycerin (UNII: PDC6A3C0OX) Polyethylene Glycol, Unspecified (UNII: 3WJQ0SDW1A) Sodium Lauryl Sulfate (UNII: 368GB5141J) Titanium Dioxide (UNII: 15FIX9V2JP) Carboxymethylcellulose Sodium (UNII: K679OBS311) Saccharin Sodium (UNII: SB8ZUX40TY) Tricalcium Phosphate (UNII: K4C08XP666) Product Characteristics Color Score Shape Size Flavor MINT (MINT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48878-3150-1 1 in 1 BOX 12/20/2010 1 113 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:48878-3150-2 1 in 1 BOX 12/20/2010 2 24 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:48878-3150-5 1 in 1 BOX 12/20/2010 01/31/2022 3 90 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 12/20/2010 Labeler - Solventum US OpCo LLC (801390852) Establishment Name Address ID/FEI Business Operations Sheffield Pharmaceuticals LLC 151177797 MANUFACTURE(48878-3150)