Label: RELIEF- sodium phosphate, monobasic and sodium phosphate, dibasic, heptahydrate enema
- NDC Code(s): 69626-0084-6
- Packager: Leosons Overseas Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 18, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
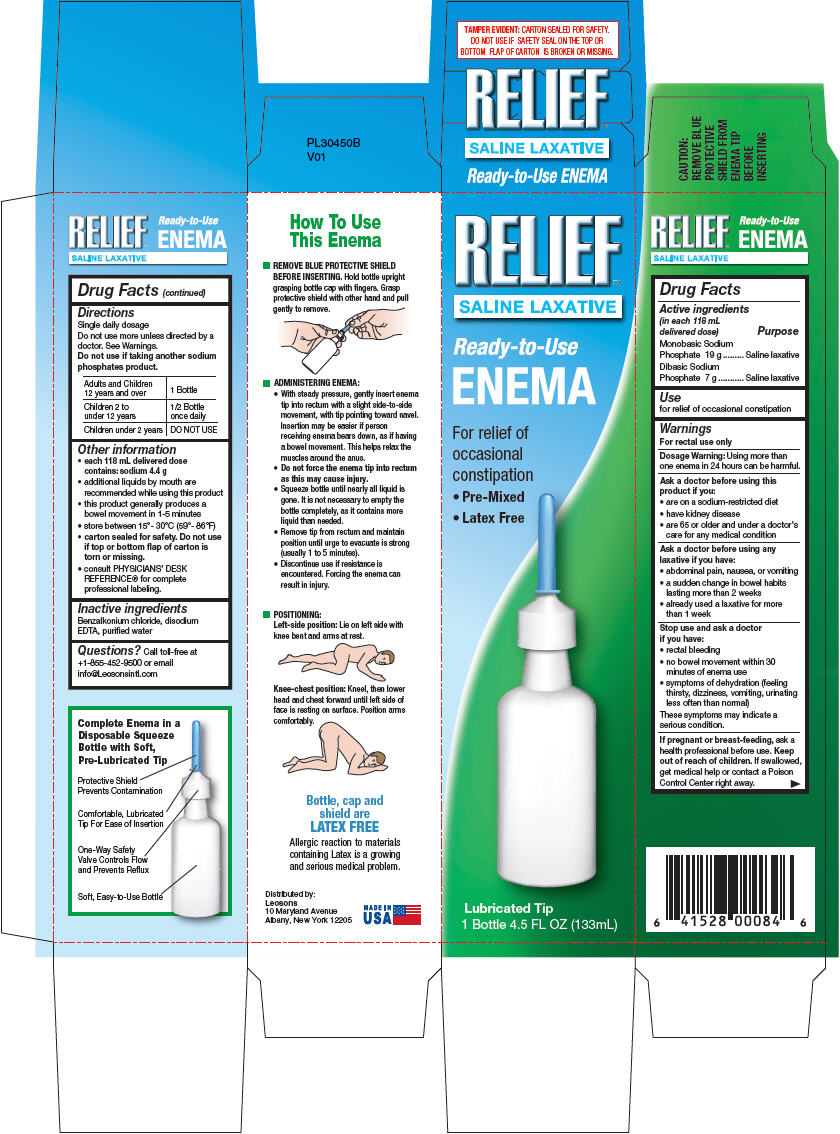
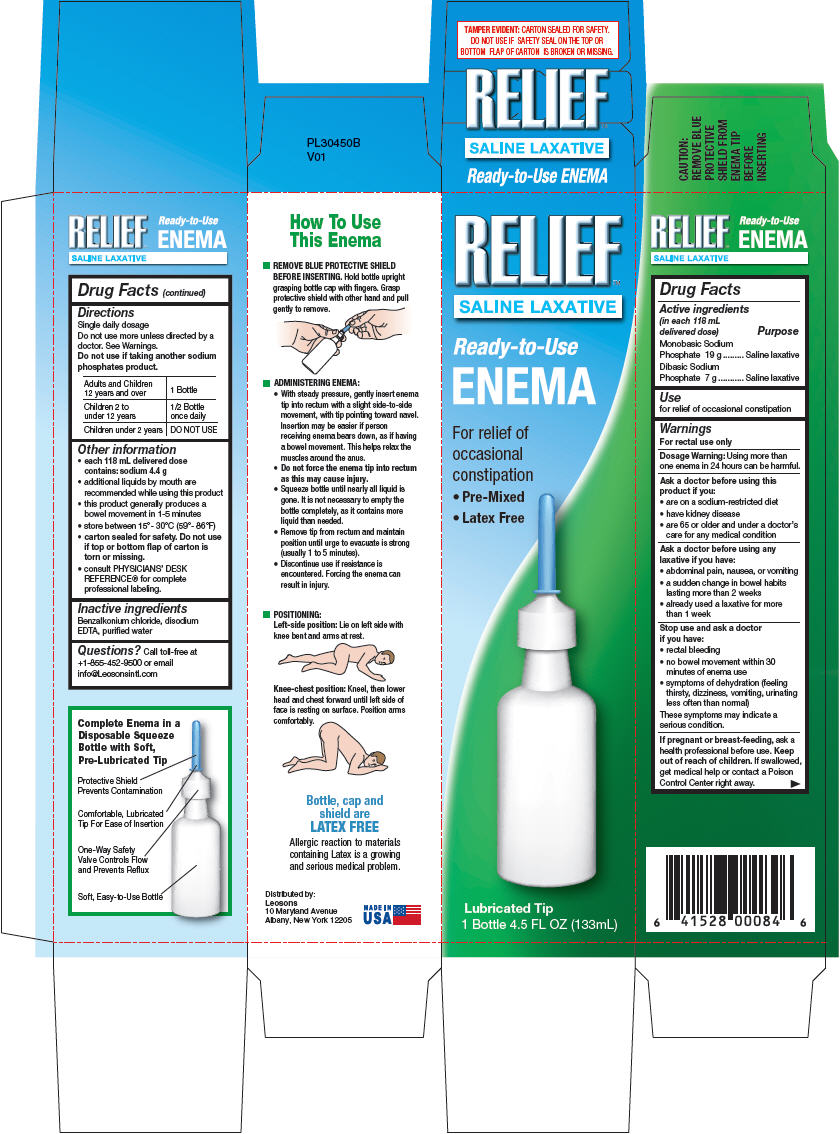
- Drug Facts
- Active ingredients & Purpose
- Use
-
Warnings
For rectal use only
Ask a doctor before using this product if you
- are on a sodium-restricted diet
- have kidney disease
- are 65 or older and under a doctor's care for any medical condition
Ask a doctor before using any laxative if you have
- abdominal pain, nausea, or vomiting
- a sudden change in bowel habits lasting more than 2 weeks
- already used a laxative for more than 1 week
-
Directions
- Single daily dosage.
- Do not use more unless directed by a doctor. See Warnings.
- Do not use if taking another sodium phosphates product.
Adults and Children 12 years and over 1 Bottle Children 2 to under 12 years 1/2 Bottle once daily Children under 2 years DO NOT USE -
Other information
- each 118 mL delivered dose contains: sodium 4.4 g
- additional liquids by mouth are recommended while using this product
- this product generally produces a bowel movement in 1-5 minutes
- store between 15°- 30°C (59°- 86°F)
- carton sealed for safety. Do not use if top or bottom flap of carton is torn or missing.
- consult PHYSICIANS' DESK REFERENCE® for complete professional labeling.
- Inactive ingredients
- Questions?
- Distributed by:
- PRINCIPAL DISPLAY PANEL - 133 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
RELIEF
sodium phosphate, monobasic and sodium phosphate, dibasic, heptahydrate enemaProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69626-0084 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC 19 g in 133 mL SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) (PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE 7 g in 133 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69626-0084-6 1 in 1 CARTON 02/09/2015 1 133 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 02/09/2015 Labeler - Leosons Overseas Corp (148605470)