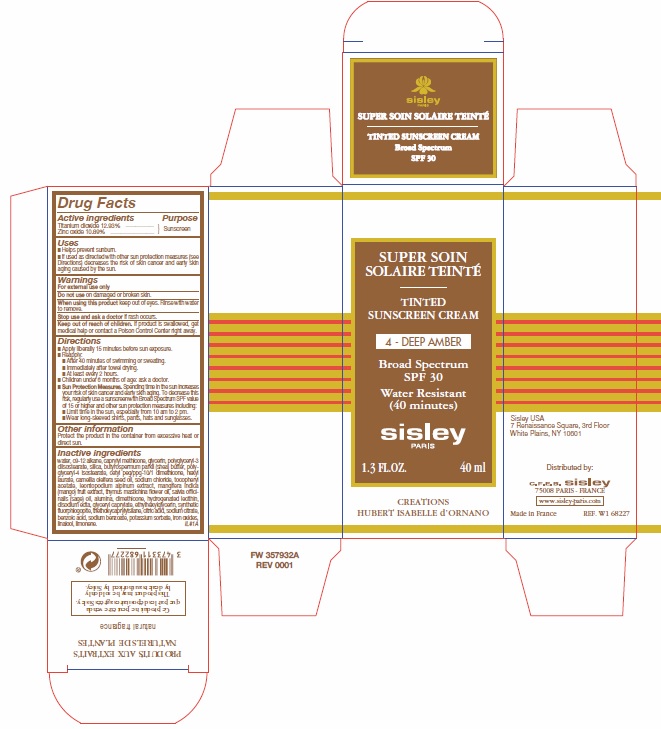
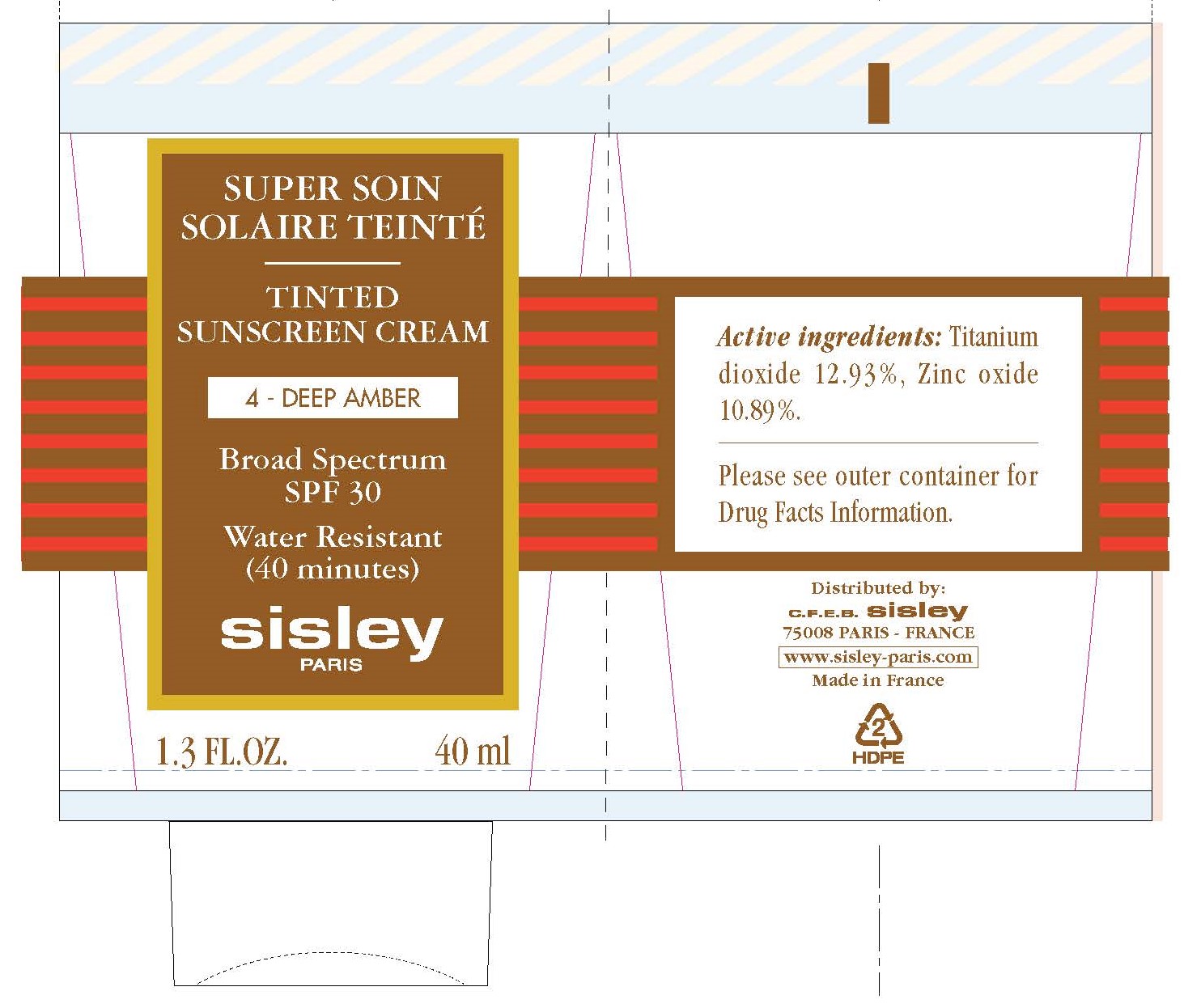
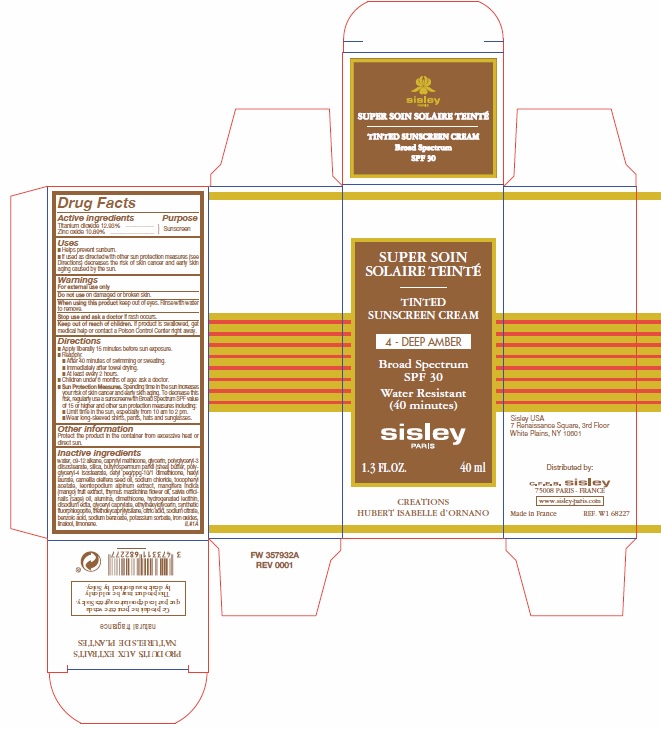
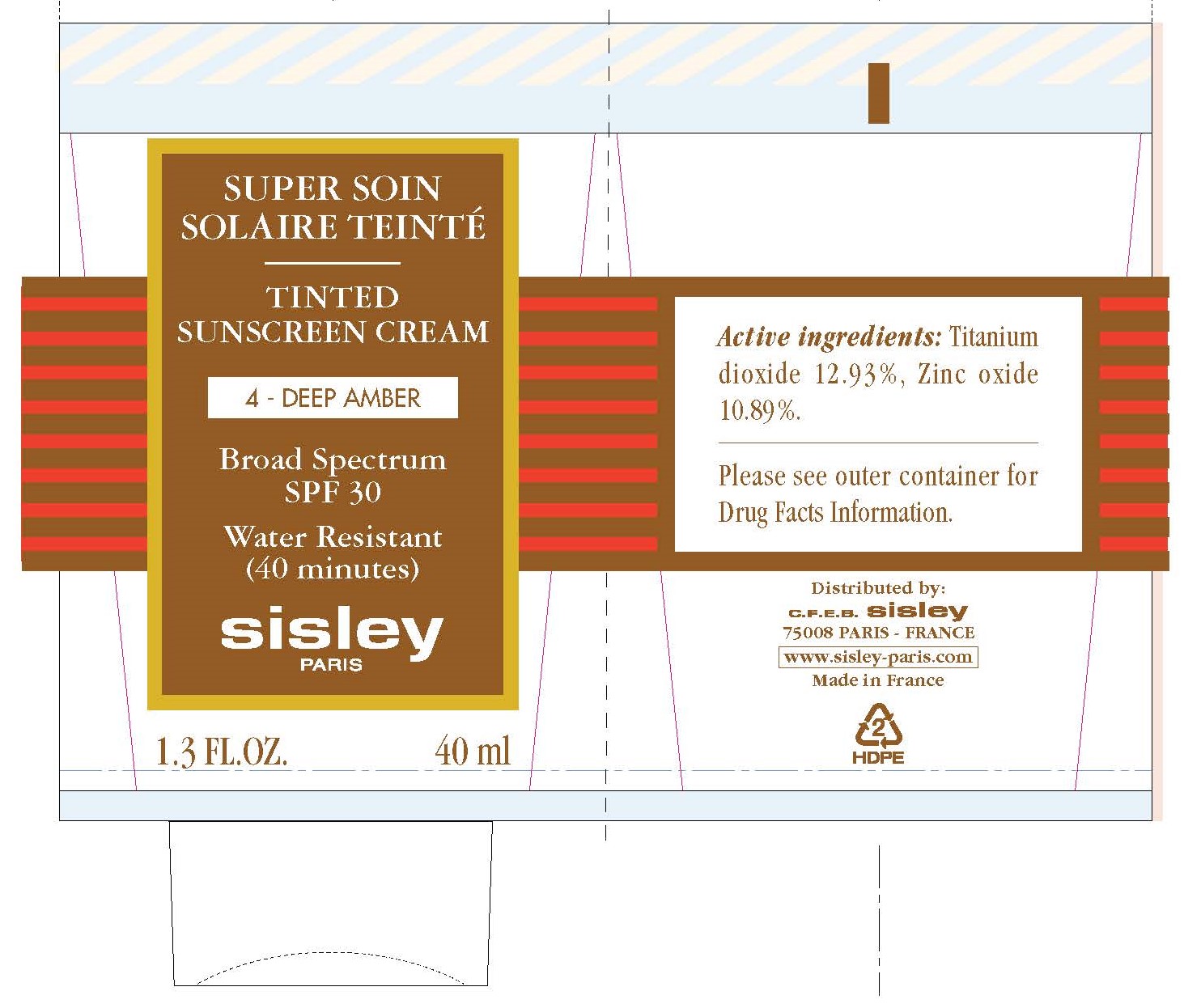
Label: TINTED SUNSCREEN CREAM SPF 30 4-DEEP AMBER- titanium dioxide, zinc oxide cream
- NDC Code(s): 66097-009-40
- Packager: C.F.E.B. - Sisley
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply:
- After 40 minutes of swimming or sweating.
- Immediately after towel drying.
- At least every 2 hours.
- Children under 6 months of age: ask a doctor.
- Spending time in the sun increases your risk of skin cancern and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Sun Protection Measures.
- Limit time in the sun, especially from 10 am to 2 pm.
- Wear long-sleeved shirts, pants, hats and sunglasses.
- Other information
-
Inactive ingredients
water, c9- 12 alkane, caprylyl methicone, glycerin, polyglyceryl-3 diisostearate, silica, butyrospermum parkii (shea) butter, polyglyceryl-4 isostearate, cetyl peg/ppg-10/1 dimethicone, hexyl laurate, camellia oleifera seed oil, sodium chloride, tocopheryl acetate, leontopodium alpinum extract, mangifera indica (mango) fruit extract, thymus mastichina flower oil, salvia officinalis (sage) oil, alumina, dimethicone, hydrogenated lecithin, disodium edta, glyceryl caprylate, ethylhexyglycerin, synthetic fluorphlogopite, triethoxycaprylylsilane, citric acid, sodium citrate, benzoic acid, sodium benzoate, potassium sorbate, iron oxides, linalool, limonene.
- Package labeling
-
INGREDIENTS AND APPEARANCE
TINTED SUNSCREEN CREAM SPF 30 4-DEEP AMBER
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66097-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 129.3 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 108.9 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) GLYCERIN (UNII: PDC6A3C0OX) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) CAMELLIA OIL (UNII: T1PE06G0VE) SODIUM CHLORIDE (UNII: 451W47IQ8X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MANGO (UNII: I629I3NR86) THYMUS MASTICHINA FLOWERING TOP OIL (UNII: 9NP0832457) SAGE OIL (UNII: U27K0H1H2O) ALUMINUM OXIDE (UNII: LMI26O6933) DIMETHICONE (UNII: 92RU3N3Y1O) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERYL MONOCAPRYLATE (UNII: TM2TZD4G4A) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) BENZOIC ACID (UNII: 8SKN0B0MIM) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66097-009-40 1 in 1 BOX 03/15/2017 1 40 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/15/2017 Labeler - C.F.E.B. - Sisley (262279246)