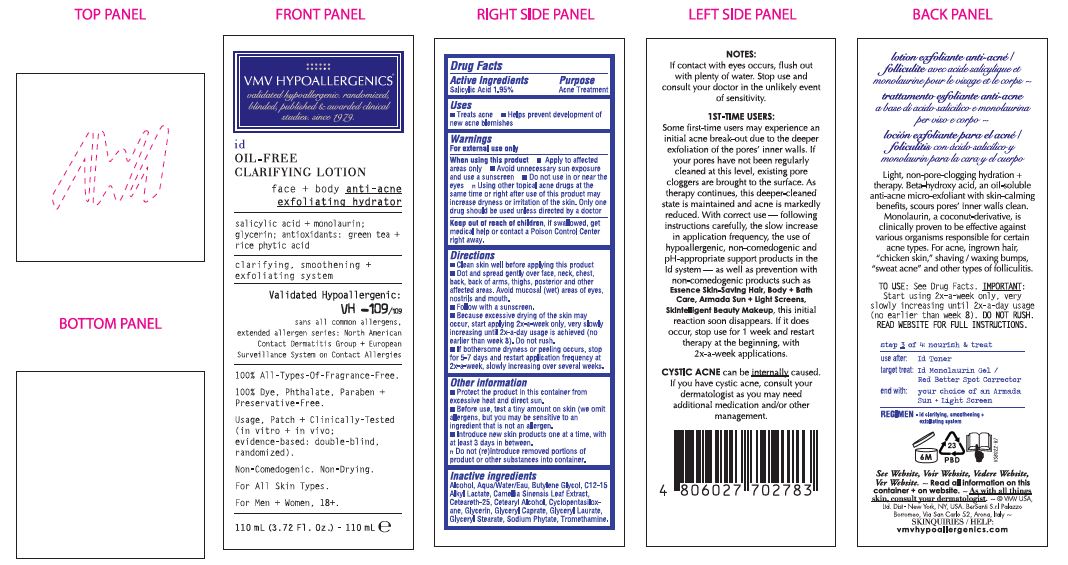
Label: ID ANTI ACNE- salicylic acid lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 69774-553-04 - Packager: SKIN SCIENCES LABORATORY INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 23, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
When using lhis product
• Apply to affected areas only
• Avoid unnecessary sun exposure and use a sunscreen
• Do not use in or near the eyes.
Using other topical acne drugs at the same time
or right after use of this product
may increase dryness or irritation of the skin.
Only one drug should be used unless directed by a doctor - KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
• Clean Directions skin well before applying this product
• Dot and spread gently over face, neck, chest, back, back of arms, thighs, posterior and other affected areas. Avoid mucosaI (wet) areas of eyes, nostrils and mouth.
• Follow with a sunscreen.
• Because excessive drying of the skin may occur, start applying 2x-a-week only, very slowly increasing until 2x-a-day usage is achieved (no earlier than week 8). Do not rush.If bothersome dryness or peeling occurs, stop for 5-7 days and restart application frequency at 2x-a-week, slowly increasing over several weeks.
-
STORAGE AND HANDLING
Other information
- Protect the product in this container from excessive heat and direct sun.
- Before use, test a tiny amount on skin (we omit allergens, but you may be sensitive
to an ingredient that is not an allergen.
- Introduce new skin products one at a time, with at least 3 days in between.
- Do not (re)introduce removed portions of product or other substances into container. - INACTIVE INGREDIENT
-
OTHER SAFETY INFORMATION
Notes: If contact with eyes
occurs, flush out with plenty of
water. This product is meant to be washed off quickly, not left on the
skin for longer than a few seconds
while cleansing. Prolonged
exposure without rinsing
immediately may cause irritation to
skin. Stop use and consult your
doctor in the unlikely event of
discomfort or sensitivity. 1ST-TIME
USERS: Some first-time users may
experience an initial acne
break-out due to the deeper
exfoliation of the pores' inner
walls. If your pores have not been
regularly cleaned at this level,
existing pore cloggers are brought
to the surface. As therapy
continues, this deeper-cleaned
state is maintained and acne is
markedly reduced. With correct
use - following instructions
carefully, the slow increase in
application frequency, the use of
hypoallergenic, non-comedogenic
and pH-appropriate support
products in the Id system - as well
as prevention with non-comedo-
genic products such as Essence
Skin-Saving Hair, Body + Bath Care,
Armada Sun + Light Screens,
Skintelligent Beauty Makeup, this
initial reaction soon disappears. If it
does occur, stop use for 1 week
and restart therapy at the
beginning, with 2x-a-week
applications. CYSTIC ACNE can
be internally caused. If you have
cystic acne, consult your
dermatologist as you may need
additional medication and/or other
management. -
PRINCIPAL DISPLAY PANEL
VMV HYPOALLERGENICS
validated hypoallergenic.
randomized, blinded, published &
awarded clinical studies. since 1979id
OIL-FREE
CLARIFYING LOTIONface + body anti-acne
exfoliating hydratorsalicylic acid+ monolaurin: glycerin; antioxidants: green tea+ rice phytic acid
clarifying, smoothening+ exfoliating systemValidated
Hypoallergenic:
VH -109/109
sans all common allergens,
extended allergen series:
North American Contact Dermatitis
Group + European Surveillance
System on Contact Allergies100% All-Types-Of-Fragrance-Free.
100% Dye, Phthalate, Paraben + Preservative-Free.
Usage, Patch + Clinically-Tested (in vitro + in vivo; evidence-based:
double-blind, randomized).Non-Comedogenic. Non-Drying.
For All Skin Types.
For Men + Women, 18+.
110 mL (3.72 Fl. Oz.) - 110 mL
-
INGREDIENTS AND APPEARANCE
ID ANTI ACNE
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69774-553 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2.15 g in 110 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) GLYCERYL CAPRATE (UNII: 197M6VFC1W) PHYTATE SODIUM (UNII: 88496G1ERL) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETEARETH-25 (UNII: 8FA93U5T67) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERYL LAURATE (UNII: Y98611C087) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) TROMETHAMINE (UNII: 023C2WHX2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69774-553-04 1 in 1 BOX 04/15/2017 1 110 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 04/15/2017 Labeler - SKIN SCIENCES LABORATORY INC. (718777360) Establishment Name Address ID/FEI Business Operations SKIN PRESCRIPTIVES MARKETING, INC. 718955750 manufacture(69774-553)