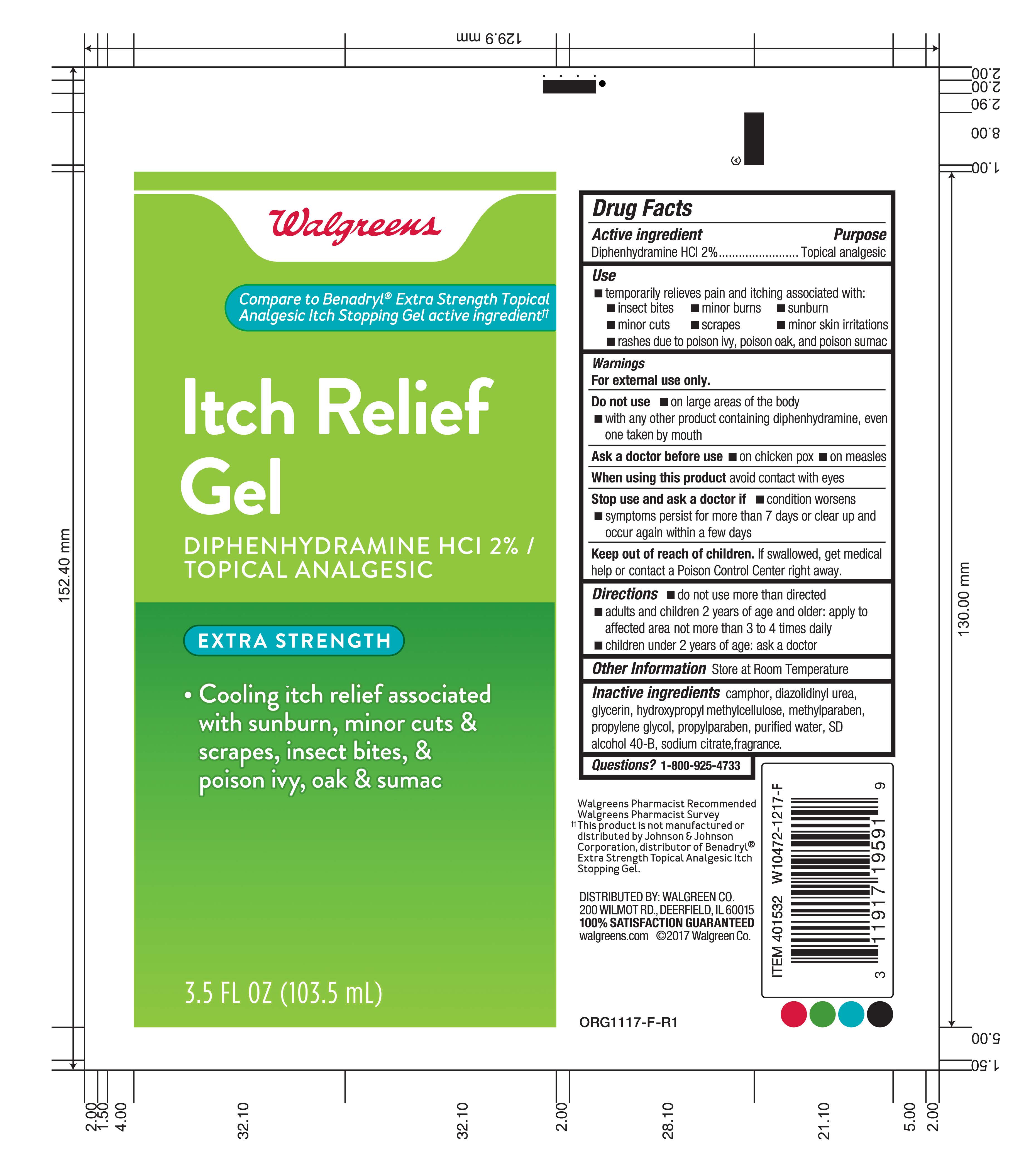
Label: ITCH RELIEF GEL- diphenhydramine hcl gel
- NDC Code(s): 0363-0353-04
- Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
- Do not use:
- Ask a doctor before use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions?
- Directions
- Tube label
-
INGREDIENTS AND APPEARANCE
ITCH RELIEF GEL
diphenhydramine hcl gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-0353 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) SODIUM CITRATE (UNII: 1Q73Q2JULR) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) ALCOHOL (UNII: 3K9958V90M) HYPROMELLOSE 2208 (15000 MPA.S) (UNII: Z78RG6M2N2) Product Characteristics Color white (clear) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-0353-04 103.5 mL in 1 TUBE; Type 0: Not a Combination Product 05/30/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/30/2018 Labeler - Walgreens (008965063) Registrant - Unipack LLC (116015769) Establishment Name Address ID/FEI Business Operations Unipack LLC 009248480 manufacture(0363-0353)