Label: MECLIZINE HYDROCHLORIDE tablet
-
Contains inactivated NDC Code(s)
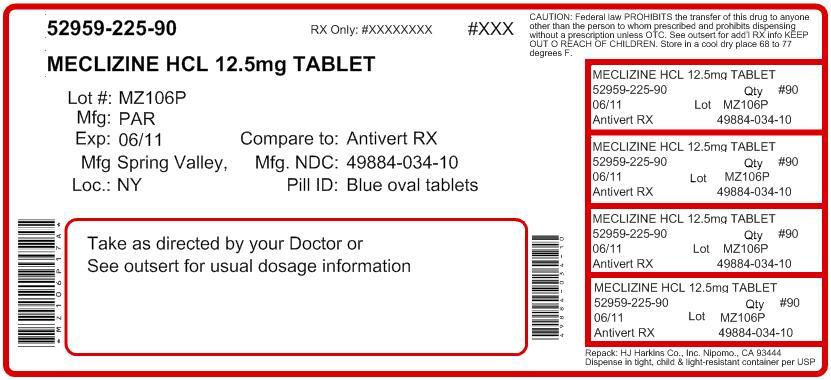
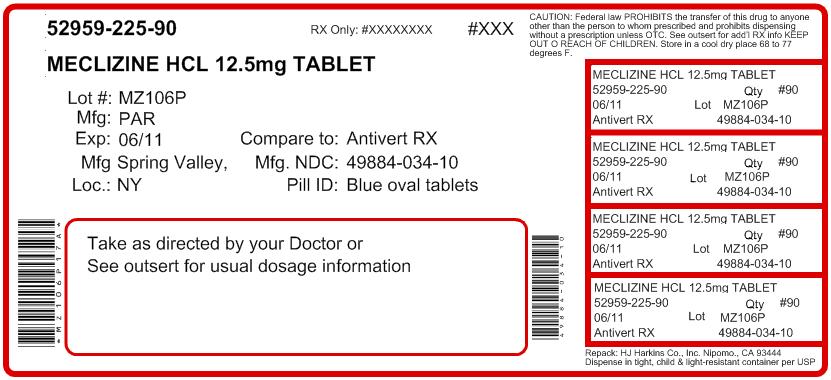
NDC Code(s): 52959-225-00, 52959-225-07, 52959-225-12, 52959-225-20, view more52959-225-21, 52959-225-30, 52959-225-60, 52959-225-90 - Packager: H.J. Harkins Company, Inc.
- This is a repackaged label.
- Source NDC Code(s): 49884-034
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 19, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
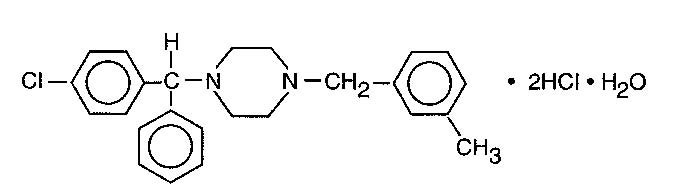
Meclizine hydrochloride, an oral antiemetic, is a white, slightly yellowish, crystalline powder which has a slight odor and is tasteless. It has the following structural formula:
C25H27CIN2•2HCI•H2O M.W. 481.89
The chemical name is 1-(p-chloro-alpha-phenylbenzyl)-4-(m-methyl-benzyl) - piperazine dihydrochloride monohydrate.
Meclizine Hydrochloride Tablets are available in 12.5 mg, and *25 mg strengths for oral administration.
*Contains FD&C Yellow #5 (see PRECAUTIONS).
Each tablet contains the following inactive ingredients: colloidal silicon dioxide, lactose, magnesium stearate, microcrystalline cellulose, sodium starch glycolate, starch, and stearic acid. In addition, the 12.5 mg tablet contains FD&C Blue #1; and the 25 mg tablet contains D&C Yellow #10 and FD&C Yellow #5.
-
CLINICAL PHARMACOLOGY
Meclizine hydrochloride is an antihistamine which shows marked protective activity against nebulized histamine and lethal doses of intravenously injected histamine in guinea pigs. It has a marked effect in blocking the vasodepressor response to histamine, but only a slight blocking action against acetylcholine. Its activity is relatively weak in inhibiting the spasmogenic action of histamine on isolated guinea pig ileum.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Since drowsiness may, on occasion, occur with use of this drug, patients should be warned of this possibility and cautioned against driving a car or operating dangerous machinery.
Patients should avoid alcoholic beverages while taking the drug. Due to its potential anticholinergic action, this drug should be used with caution in patients with asthma, glaucoma, or enlargement of the prostate gland. Do not give to children under 12 years of age unless directed by a doctor.
-
PRECAUTIONS
The Meclizine Hydrochloride Tablets, 25 mg contain FD&C Yellow #5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible individuals. Although the overall incidence of FD&C Yellow #5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Usage in Children: Clinical studies establishing safety and effectiveness in children have not been done; therefore, usage is not recommended under 12 years of age.
Usage in Pregnancy:Pregnancy Category B. Reproduction studies in rats have shown cleft palates at 25-50 times the human dose. Epidemiological studies in pregnant women, however, do not indicate that meclizine hydrochloride increases the risk of abnormalities when administered during pregnancy.
Despite the animal findings, it would appear that the possibility of fetal harm is remote. Nevertheless, meclizine hydrochloride, or any other medication should be used during pregnancy only if clearly necessary.
- ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Meclizine Hydrochloride Tablets, USP 12.5 mg - blue, oval tablets debossed with “034” on one side and “par” on the other side. Tablets may contain characteristic dye spots. They are supplied in bottles of 100 (NDC 49884-034-01) and 1000 (NDC 49884-034-10).
Meclizine Hydrochloride Tablets, USP 25 mg - yellow, oval tablets debossed with “035” on one side and “par” on the other side. They are supplied in bottles of 100 (NDC 49884-035-01) and 1000 (NDC 49884-035-10).
Dispense in tight, light-resistant containers as defined in the USP.
Store at controlled room temperature 15°-30°C (59°-86°F).
Manufactured by:
PAR PHARMACEUTICAL COMPANIES, INC.
Spring Valley, NY 10977
Repacked by:
H.J. Harkins Company, Inc.
Nipomo, CA 93444
Revised: 11/11
OS034-01-1-11
- PRINCIPAL DISPLAY PANEL: 12.5 MG CONTAINER LABEL 100 TABLETS
-
INGREDIENTS AND APPEARANCE
MECLIZINE HYDROCHLORIDE
meclizine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52959-225(NDC:49884-034) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECLIZINE HYDROCHLORIDE (UNII: HDP7W44CIO) (MECLIZINE - UNII:3L5TQ84570) MECLIZINE HYDROCHLORIDE 12.5 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color BLUE Score no score Shape OVAL Size 5mm Flavor Imprint Code Par;034 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52959-225-00 100 in 1 BOTTLE 2 NDC:52959-225-07 7 in 1 BOTTLE 3 NDC:52959-225-12 12 in 1 BOTTLE 4 NDC:52959-225-20 20 in 1 BOTTLE 5 NDC:52959-225-21 21 in 1 BOTTLE 6 NDC:52959-225-30 30 in 1 BOTTLE 7 NDC:52959-225-60 60 in 1 BOTTLE 8 NDC:52959-225-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA087127 06/03/1981 Labeler - H.J. Harkins Company, Inc. (147681894) Registrant - H.J. Harkins Company, Inc. (147681894) Establishment Name Address ID/FEI Business Operations H.J. Harkins Company, Inc. 147681894 repack, relabel