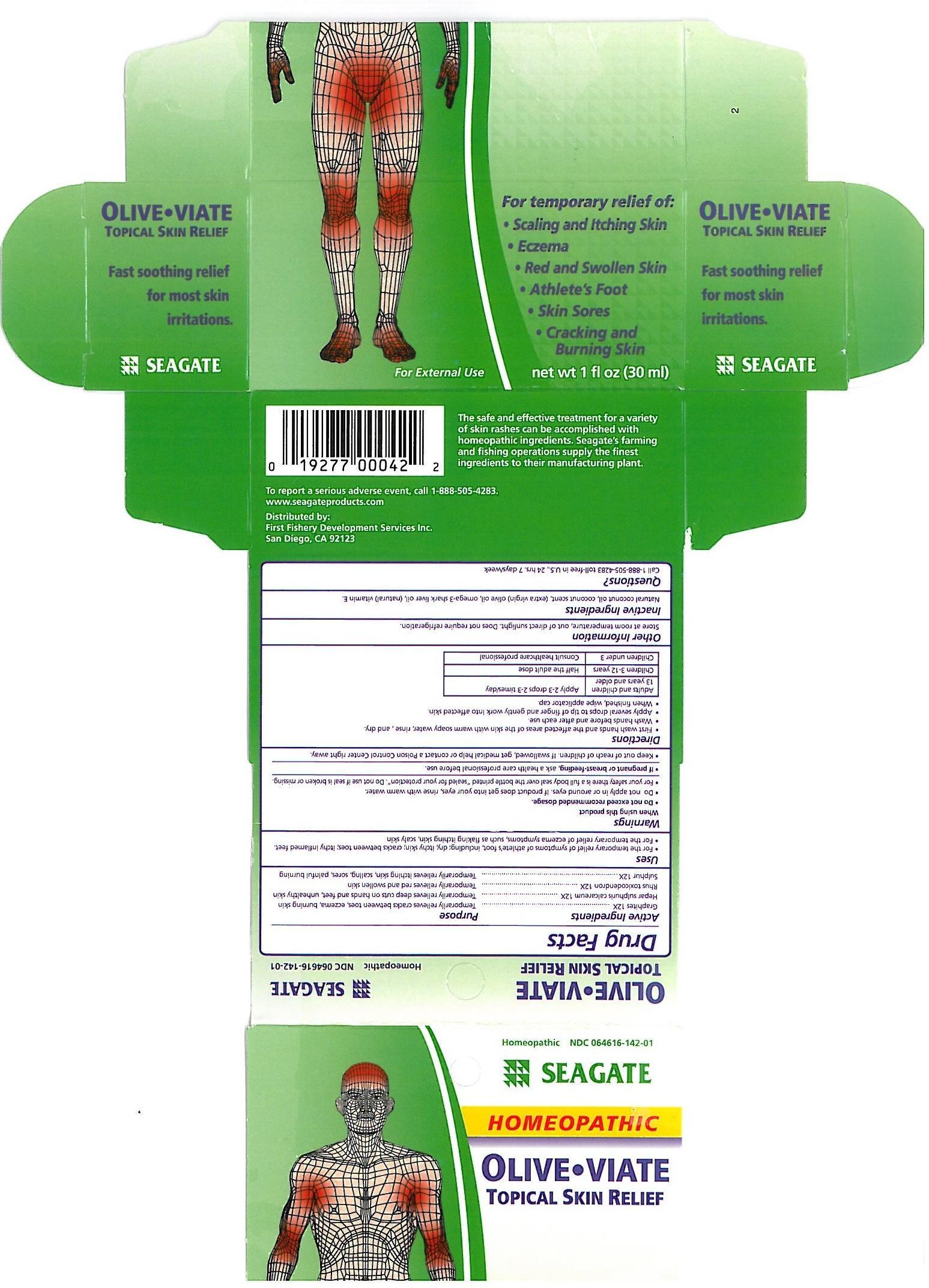
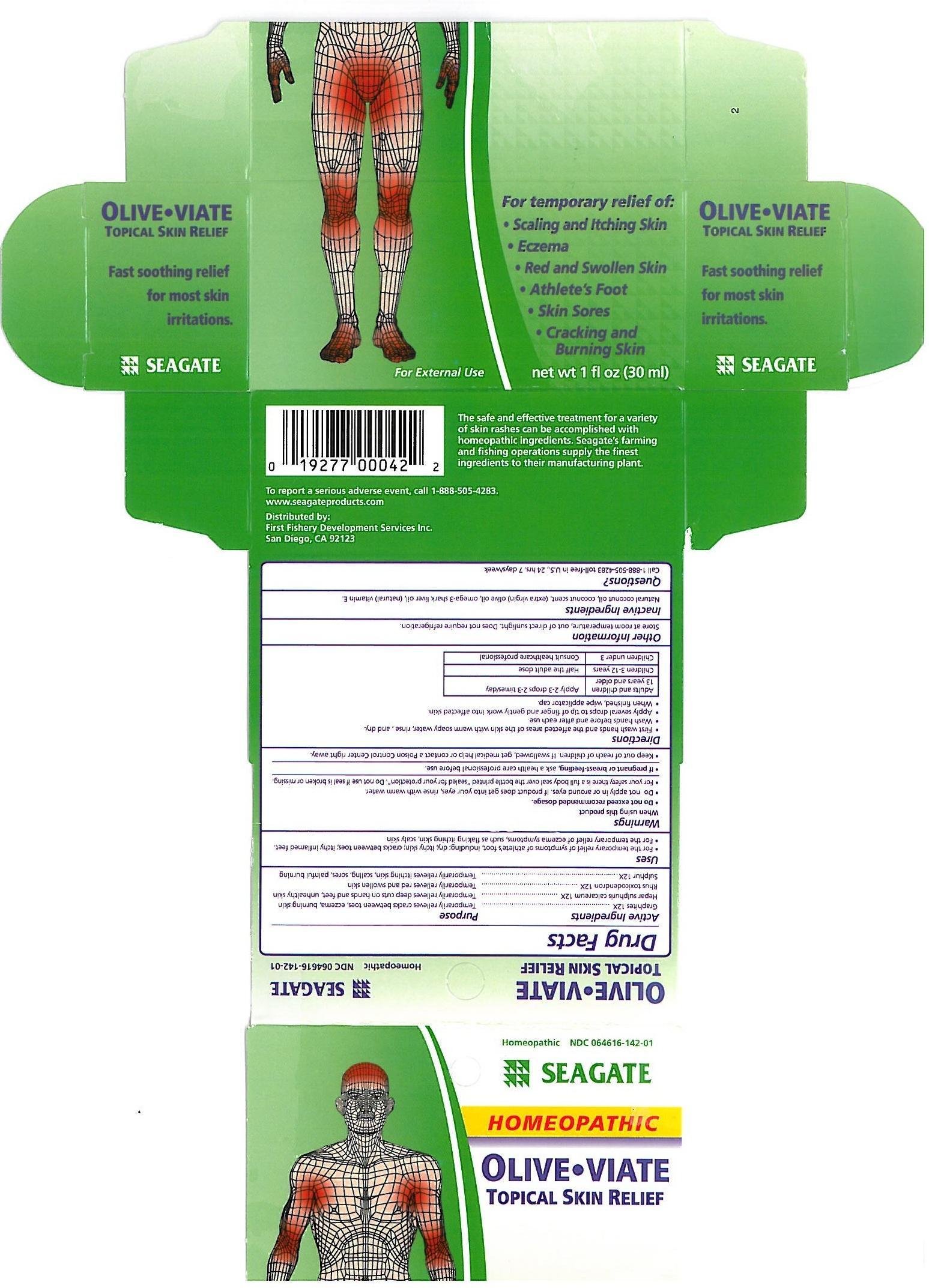
Label: OLIVE-VIATE- topical skin relief liquid
- NDC Code(s): 64616-042-01, 64616-042-02
- Packager: Vitality Works, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Olive-Viate
- Olive-Viate
- Olive-Viate
- Olive-Viate
- Olive-Viate
-
Olive-Viate
First wash hands and the affected areas of the skin with warm soapy water, rinse, and dry.
Wash hands before and after each use.
Apply several drops to tip of finger and gently work into affected skin.
When finished, wipe applicator cap.
Adults and children 13 years and older: Apply 1-3 drops 2-3 times a day.
Children 3-12 years: Half the adult dose.
Childern under 3: Consult healthcare professional.
-
Olive-Viate
Graphites - temporarily relieves cracks between toes, eczema burning skin.
Hepar sulphuris calcareum - temporily relieves deep cuts on hands and feet, unhealthy skin.
Rhus toxicodendron - Temporarily relieves red and swollen skin.
Sulphur - temporarily relieves itchy skin, scaling sores, painful burning.,
- Olive-Viate
-
INGREDIENTS AND APPEARANCE
OLIVE-VIATE
topical skin relief liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64616-042 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 12 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 12 [hp_X] in 1 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 12 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength COCONUT OIL (UNII: Q9L0O73W7L) OLIVE OIL (UNII: 6UYK2W1W1E) SHARK LIVER OIL (UNII: 4B24275HEU) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64616-042-02 1 in 1 CARTON 03/02/2004 1 NDC:64616-042-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/02/2004 Labeler - Vitality Works, Inc. (137752817) Registrant - Vitality Works, Inc. (137752817) Establishment Name Address ID/FEI Business Operations Vitality Works, Inc. 137752817 manufacture(64616-042)