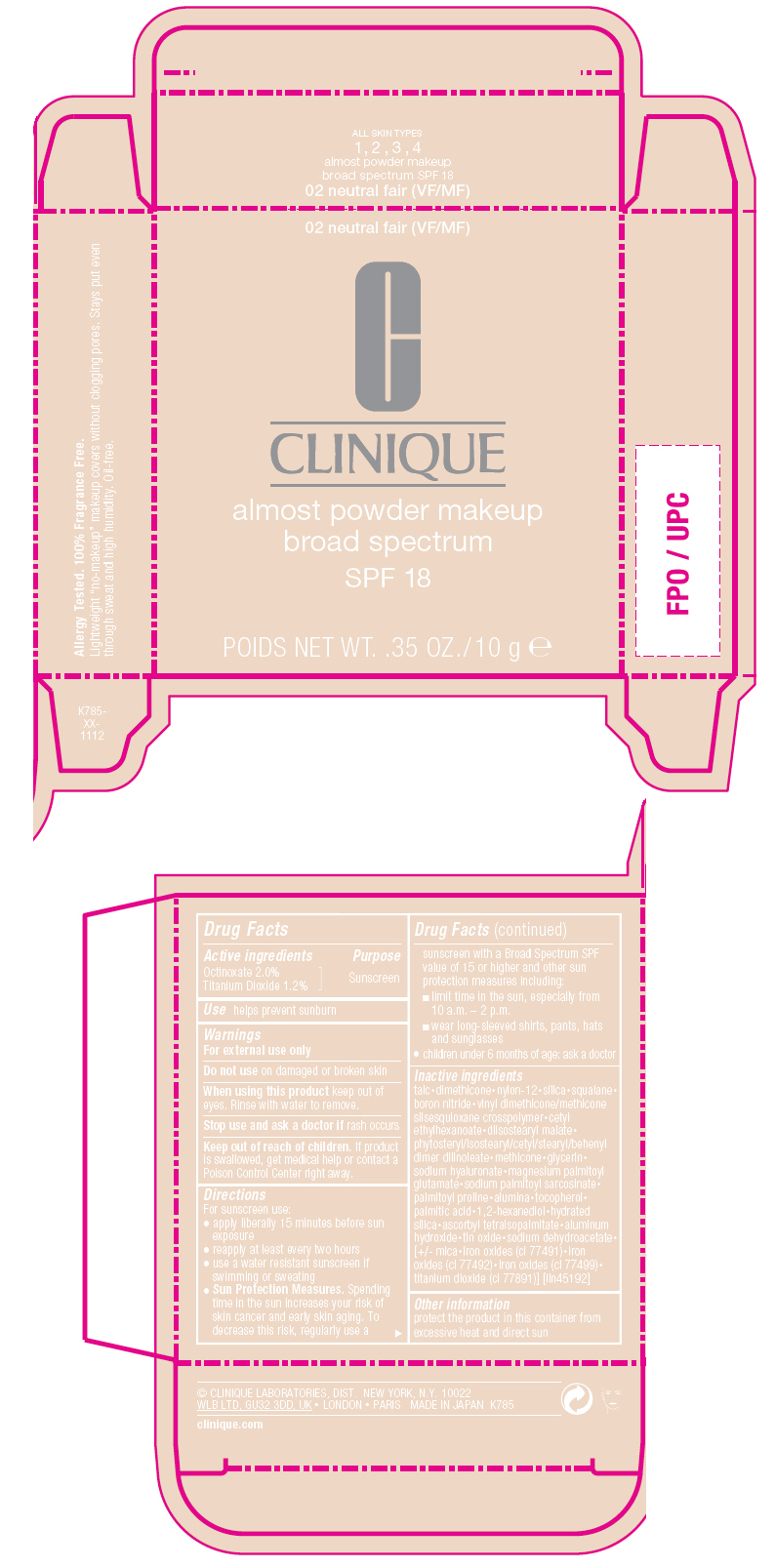
Label: ALMOST POWDER MAKEUP BROAD SPECTRUM SPF 18- octinoxate and titanium dioxide powder
- NDC Code(s): 49527-060-01
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
talc•dimethicone•nylon-12•silica•squalane•boron nitride•vinyl dimethicone/methicone silsesquioxane crosspolymer•cetyl ethylhexanoate•diisostearyl malate•phytosteryl/isostearyl/cetyl/stearyl/behenyl dimer dilinoleate•methicone•glycerin•sodium hyaluronate•magnesium palmitoyl glutamate•sodium palmitoyl sarcosinate•palmitoyl proline•alumina•tocopherol•palmitic acid•1,2-hexanediol•hydrated silica•ascorbyl tetraisopalmitate•aluminum hydroxide•tin oxide•sodium dehydroacetate•[+/- mica•iron oxides (ci 77491)•iron oxides (ci 77492)•iron oxides (ci 77499)•titanium dioxide (ci 77891)] [iln45192]
- Other information
- PRINCIPAL DISPLAY PANEL - 10 g Jar Carton
-
INGREDIENTS AND APPEARANCE
ALMOST POWDER MAKEUP BROAD SPECTRUM SPF 18
octinoxate and titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-060 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.2 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) DIMETHICONE (UNII: 92RU3N3Y1O) NYLON-12 (UNII: 446U8J075B) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SQUALANE (UNII: GW89575KF9) BORON NITRIDE (UNII: 2U4T60A6YD) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) FERRIC OXIDE RED (UNII: 1K09F3G675) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) PHYTOSTERYL/ISOSTEARYL/CETYL/STEARYL/BEHENYL DIMER DILINOLEATE (UNII: 8N725H4EFN) METHICONE (20 CST) (UNII: 6777U11MKT) GLYCERIN (UNII: PDC6A3C0OX) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MAGNESIUM PALMITOYL GLUTAMATE (UNII: DH37YM1F48) SODIUM PALMITOYL SARCOSINATE (UNII: 7297LY09YF) PALMITOYL PROLINE (UNII: I49727TDYF) ALUMINUM OXIDE (UNII: LMI26O6933) TOCOPHEROL (UNII: R0ZB2556P8) PALMITIC ACID (UNII: 2V16EO95H1) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HYDRATED SILICA (UNII: Y6O7T4G8P9) ASCORBYL TETRAISOPALMITATE (UNII: 47143LT58A) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) STANNIC OXIDE (UNII: KM7N50LOS6) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) MICA (UNII: V8A1AW0880) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-060-01 1 in 1 CARTON 03/01/2018 1 10 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/01/2018 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations ASANUMA CORPORATION 715464942 manufacture(49527-060)