Label: TOOTHPOWDER- sodium fluoride powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 71035-500-01, 71035-500-02, 71035-500-03 - Packager: Yangzhou Hongshengding Chemical Co.,Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
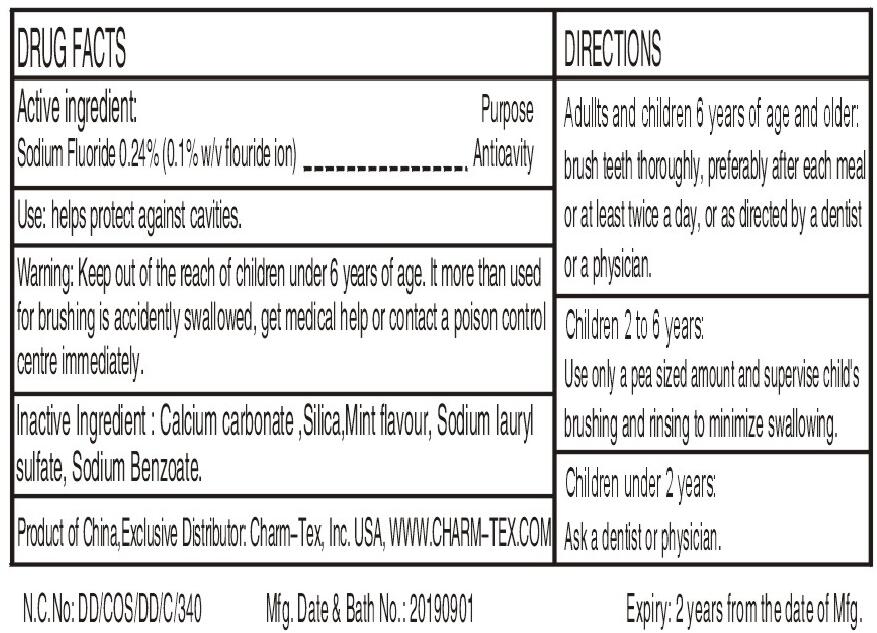
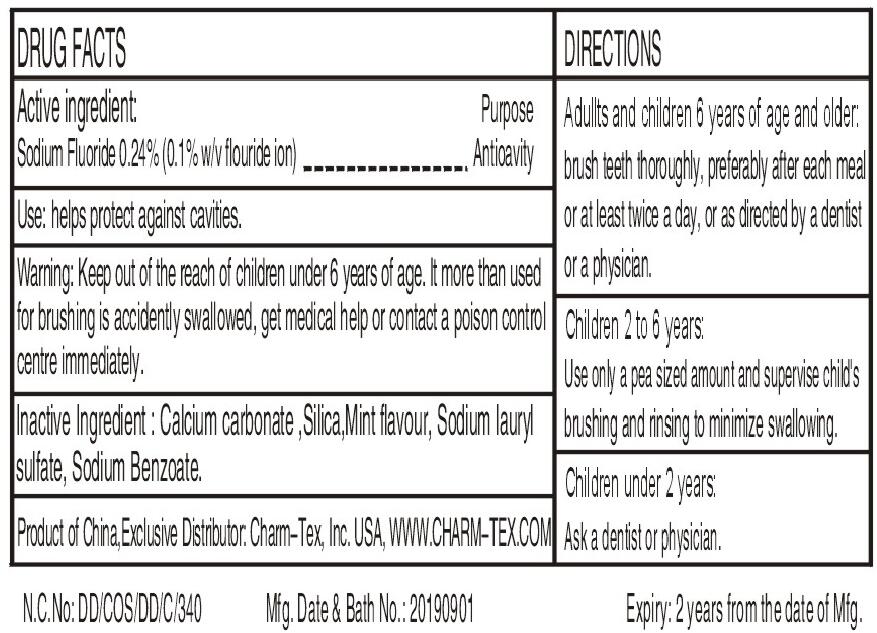
- Active ingredients
- Uses
- Warnings
-
Directions
Adults and children 2years of age and older:Brush teeth thoroughly,preferably after each meal or at least twice a day,or as directed by a dentist or a physician.
Children 2-6 years:Use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing)
Children under 2 years:Ask a dentist or a physician.
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOOTHPOWDER
sodium fluoride powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71035-500 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.24 g in 100 g Inactive Ingredients Ingredient Name Strength CALCIUM CARBONATE (UNII: H0G9379FGK) SODIUM SILICATE (UNII: IJF18F77L3) MINT (UNII: FV98Z8GITP) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71035-500-03 250 in 1 CARTON 09/09/2016 1 85 g in 1 BAG; Type 0: Not a Combination Product 2 NDC:71035-500-02 250 in 1 CARTON 09/09/2016 2 57 g in 1 BAG; Type 0: Not a Combination Product 3 NDC:71035-500-01 250 in 1 CARTON 09/09/2016 3 28 g in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 09/04/2016 Labeler - Yangzhou Hongshengding Chemical Co.,Ltd. (543774755) Registrant - Yangzhou Hongshengding Chemical Co.,Ltd. (543774755) Establishment Name Address ID/FEI Business Operations Yangzhou Hongshengding Chemical Co.,Ltd. 543774755 manufacture(71035-500)