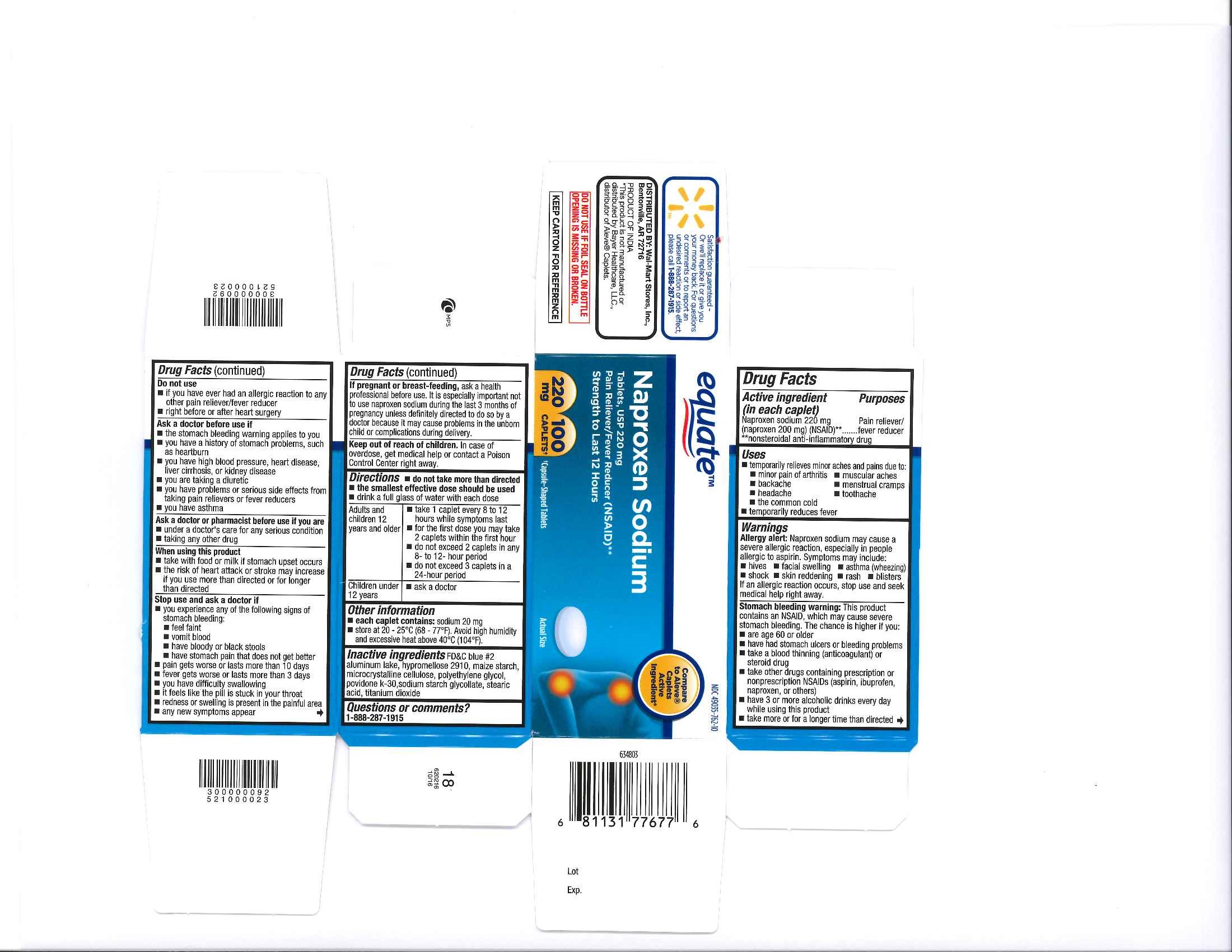
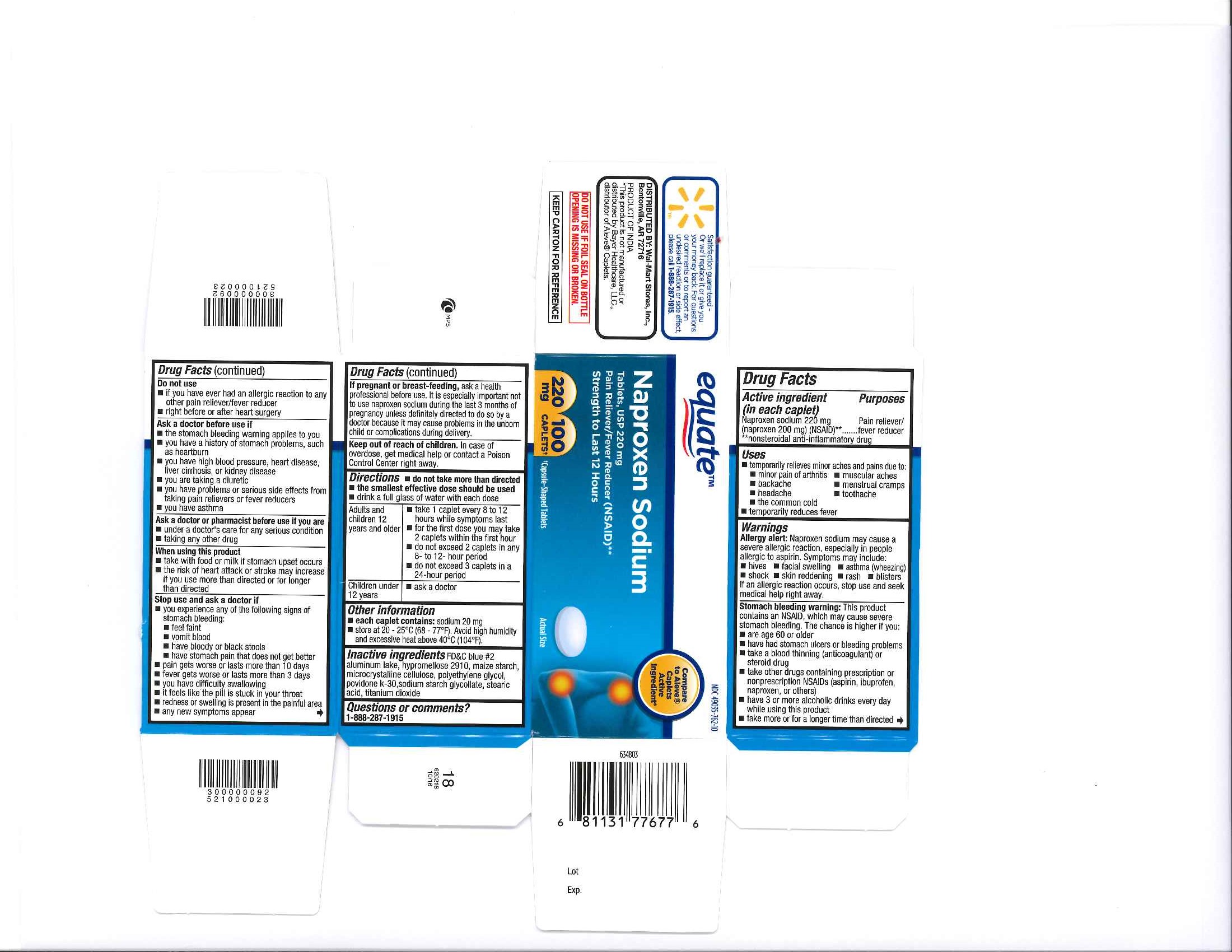
Label: NAPROXEN SODIUM tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 49035-761-21, 49035-762-10, 49035-762-21, 49035-762-24, view more49035-762-30 - Packager: Wal-Mart Stores, Inc.,
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 6, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Allergy alert
-
Stomach bleeding warning
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- Do not use
-
Ask a doctor before use if
- the stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
- you have problems or serious side effects from taking pain relievers or fever reducers
- you have asthma
- Ask a doctor or pharmacist before use if you are
- When using this product
-
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding
■ feel faint
■ vomit blood
■ have bloody or black stools
■ have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing
- it feels like the pill is stuck in your throat
- redness or swelling is present in the painful area
- any new symptoms appear
- If pregnant or breast-feeding
- Keep out of the reach of children
-
Directions
- do not take more than directed
- the smallest effective dose should be used
- drink a full glass of water with each dose
adults and children 12 years and older,
■ take 1 caplet every 8 to 12 hours while symptoms last
■ for the first dose you may take 2 caplets within the first hour
■ do not exceed 2 caplets in any 8- to 12- hour period
■ do not exceed 3 caplets in a 24- hour period
children under 12 years,
■ ask a doctor
- Other information
- Questions and comments?
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NAPROXEN SODIUM
naproxen sodium tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-762 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN SODIUM (UNII: 9TN87S3A3C) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN SODIUM 220 mg Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) HYPROMELLOSES (UNII: 3NXW29V3WO) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE K30 (UNII: U725QWY32X) Product Characteristics Color blue Score no score Shape OVAL Size 12mm Flavor Imprint Code 220 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-762-24 24 in 1 BOTTLE; Type 0: Not a Combination Product 05/25/2016 2 NDC:49035-762-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/25/2016 3 NDC:49035-762-21 225 in 1 BOTTLE; Type 0: Not a Combination Product 05/25/2016 4 NDC:49035-762-30 300 in 1 BOTTLE; Type 0: Not a Combination Product 05/25/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091353 05/25/2016 NAPROXEN SODIUM
naproxen sodium tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49035-761 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPROXEN SODIUM (UNII: 9TN87S3A3C) (NAPROXEN - UNII:57Y76R9ATQ) NAPROXEN SODIUM 220 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 2 (UNII: L06K8R7DQK) HYPROMELLOSES (UNII: 3NXW29V3WO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONE K30 (UNII: U725QWY32X) STEARIC ACID (UNII: 4ELV7Z65AP) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color blue Score no score Shape ROUND Size 10mm Flavor Imprint Code I1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49035-761-21 225 in 1 BOTTLE; Type 0: Not a Combination Product 05/25/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091353 05/25/2016 Labeler - Wal-Mart Stores, Inc., (051957769)