Label: THE CURE SHEER CREAM- octinoxate, titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 63730-171-01, 63730-171-02, 63730-171-03, 63730-171-04, view more63730-171-05 - Packager: Natura Bisse International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 27, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
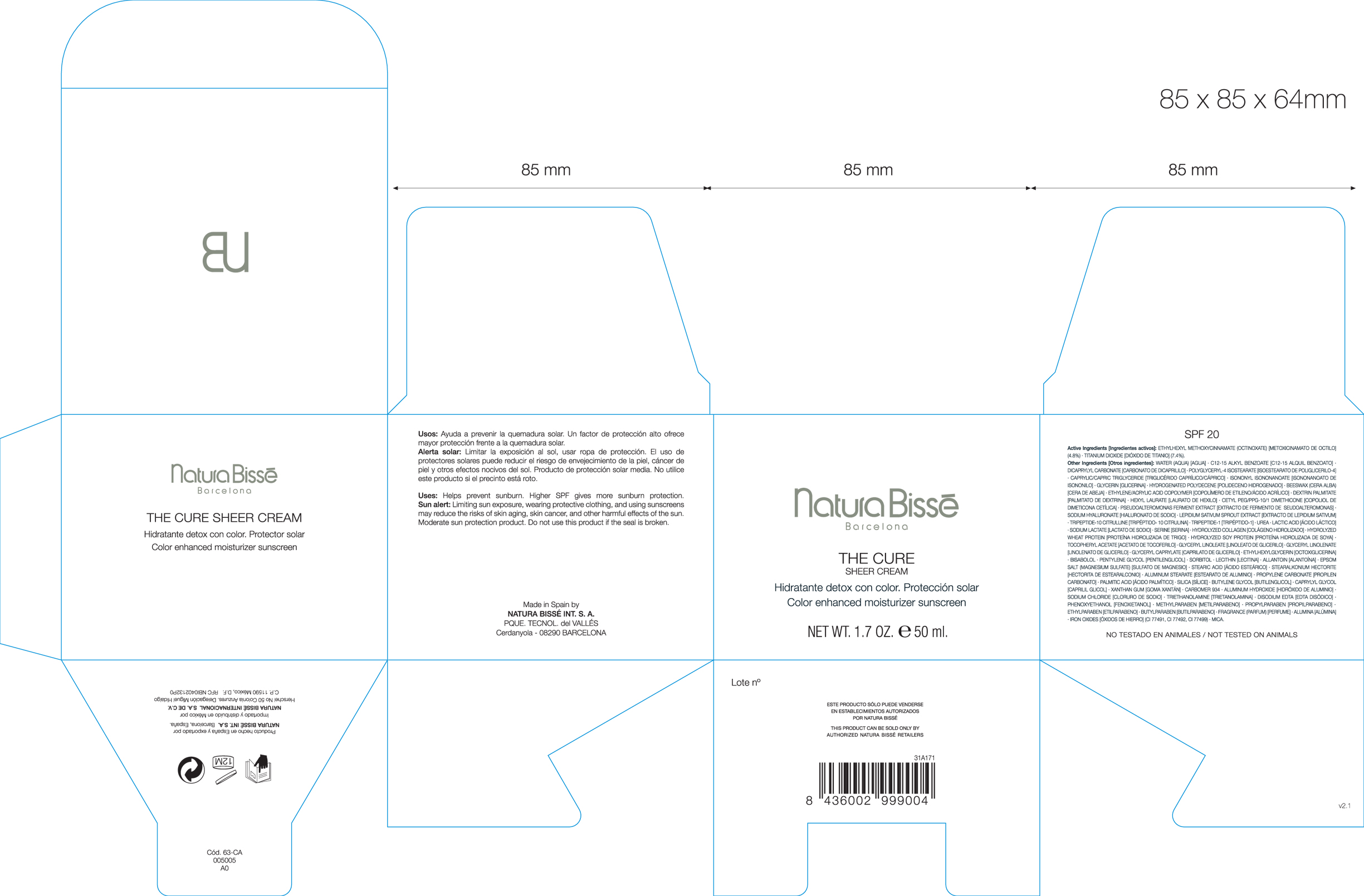
Natura Bisse Barcelona
The Cure Sheer Cream
Color Enhanced moisturizer sunscreen
NET WT. 1.7 OZ e 50 ml.
Imported and distributed in Mexico for
Natura Bisse International S.A. de C.V.
Herschel No 50 Colonia Anzures. Delegacion Miguel Hidalgo
C.P. 11590 Mexico D.F. RFC NBI0402132PO
Expires 12 Months after opening
This Product Can Be Sold Only by Authorized Natura Bisse Retailers
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Other Ingredients: Water (Aqua), C12-15 Alkyl Benzoate, Dicaprylyl Carbonate, Polyglyceryl-4 Isostearate, Caprylic/Capric Triglyceride, Isononyl Isononanoate, Glycerin, Hydrogenated Polydecene, Beeswax, Ethylene/Acrylic Acid Copolymer, Dextrin Palmitate, Hexyl Laurate, Cetyl PEG/PPG-10/1 Dimethicone, Pseudoalteromonas Ferment Extract, Sodium Hyaluronate, Lepidium Sativum Sprout Extract, Tripeptide-10 Citrulline, Tripeptide-1, Urea, Lactic Acid, Sodium Lactate, Serine, Hydrolyzed Collagen, Hydrolyzed Wheat Protein, Hydrolyzed Soy Protein, Tocopheryl Acetate, Glyceryl Linoleate, Glyceryl Linolenate, Glyceryl Caprylate, Ethylhexylglycerin, Bisabolol, Pentylene Glycol, Sorbitol, Lecithin, Allantoin, Epsom Salt (Magnesium Sulfate), Stearic Acid, Stearalkonium Hectorite, Aluminum Stearate, Propylene Carbonate, Palmitic Acid, Silica, Butylene Glycol, Caprylyl Glycol, Xanthan Gum, Carbomer 934, Aluminum Hydroxide, Sodium Chloride, Triethanolamine, Disodium EDTA, Phenoxyethanol, Methylparaben, Propylparaben, Ethylparaben, Butylparaben, Fragrance, Alumina, Iron Oxides and Mica.
Not Tested on Animals
-
INDICATIONS & USAGE
Uses: Helps prevent sunburn. Higher SPF gives more sunburn protection.
Sun alert: Limiting sun exposure, wearing protective clothing, and using sunscreens may reduce the risks of skin aging, skin cancer, and other harmful effects of the sun. Moderate sun protection product. Do not use this product if the seal is broken.
Made in Spain by
Natura Bisse Int. S.A.
PQUE. Tecnol. del Valles
Cerdanyola 08290 Barcelona
-
INGREDIENTS AND APPEARANCE
THE CURE SHEER CREAM
octinoxate, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63730-171 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 2 mL in 50 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 4 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) C12-15 Alkyl Benzoate (UNII: A9EJ3J61HQ) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Caprylic/Capric Mono/Diglycerides (UNII: U72Q2I8C85) Isononyl Isononanoate (UNII: S4V5BS6GCX) Glycerin (UNII: PDC6A3C0OX) Wax, Yellow (UNII: 2ZA36H0S2V) Hexyl Laurate (UNII: 4CG9F9W01Q) Hyaluronate Sodium (UNII: YSE9PPT4TH) Urea (UNII: 8W8T17847W) Lactic Acid (UNII: 33X04XA5AT) Sodium Lactate (UNII: TU7HW0W0QT) Serine (UNII: 452VLY9402) Ethylhexylglycerin (UNII: 147D247K3P) Sorbitol (UNII: 506T60A25R) Allantoin (UNII: 344S277G0Z) Magnesium Sulfate (UNII: DE08037SAB) Stearic Acid (UNII: 4ELV7Z65AP) Aluminum Stearate (UNII: U6XF9NP8HM) Propylene Carbonate (UNII: 8D08K3S51E) Palmitic Acid (UNII: 2V16EO95H1) Silicon Dioxide (UNII: ETJ7Z6XBU4) Butylene Glycol (UNII: 3XUS85K0RA) Caprylyl Glycol (UNII: 00YIU5438U) Xanthan Gum (UNII: TTV12P4NEE) Carbomer 934 (UNII: Z135WT9208) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Sodium Chloride (UNII: 451W47IQ8X) Trolamine (UNII: 9O3K93S3TK) Phenoxyethanol (UNII: HIE492ZZ3T) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Ethylparaben (UNII: 14255EXE39) Butylparaben (UNII: 3QPI1U3FV8) Aluminum Oxide (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63730-171-01 50 mL in 1 CARTON 2 NDC:63730-171-02 2 mL in 1 CARTON 3 NDC:63730-171-03 7 mL in 1 CARTON 4 NDC:63730-171-04 10 mL in 1 CARTON 5 NDC:63730-171-05 100 mL in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/01/2010 Labeler - Natura Bisse International (464431576) Establishment Name Address ID/FEI Business Operations Natura Bisse International 464431576 manufacture

