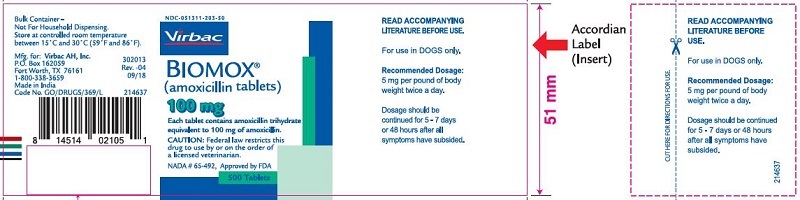
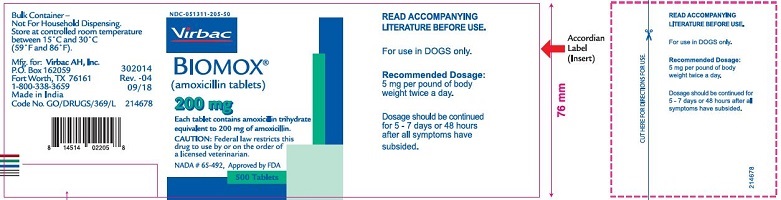
Label: BIOMOX- amoxicillin tablet
- NDC Code(s): 51311-201-50, 51311-203-50, 51311-205-50
- Packager: Virbac AH, Inc.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated January 5, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- Inactive Ingredients
-
ACTION
Amoxicillin has bactericidal activity against susceptible organisms similar to that of ampicillin. It acts by inhibiting the biosynthesis of bacterial cell wall mucopeptides. Most strains of the following grampositive and gram-negative bacteria have demonstrated susceptibility to amoxicillin, both in vitro and in vivo: nonpenicillinase-producing staphylococci, alpha- and beta- hemolytic streptococci, Enterococcus faecalis, Escherichia coli and Proteus mirabilis. Amoxicillin does not resist destruction by penicillinase; therefore, it is not effective against penicillinase-producing bacteria, particularly resistant staphylococci. Most strains of Enterobacter and Klebsiella and all strains of Pseudomonas are resistant. Amoxicillin may be given without regard to meals because it is stable in gastric acid. It is rapidly absorbed following oral administration and diffuses readily into most body fluids and tissues. It diffuses poorly into the brain and spinal fluid except when the meninges are inflamed. Most of the amoxicillin is excreted in the urine unchanged.
-
INDICATIONS
BIOMOX® (amoxicillin tablets) are indicated for treatment of the following infections in dogs when caused by susceptible strains of organisms:
BACTERIAL DERMATITIS due to Staphylococcus aureus, Strepto-coccus spp., Staphylococcus spp., and Escherichia coli.
SOFT TISSUE INFECTIONS
(abscesses, wounds, lacerations) due to Staphylococcus aureus,Enterococcus faecalis, Escherichia coli, Proteus mirabilis, and Staphylococcus spp.With all antibiotic therapy, appropriate in vitro cultures and sensitivities should be conducted prior to treatment.
- CONTRAINDICATIONS
- ADVERSE REACTIONS
- WARNINGS
- PRECAUTIONS
- CAUTION
- DOSAGE AND ADMINISTRATION
- SUPPLY
- SPL UNCLASSIFIED SECTION
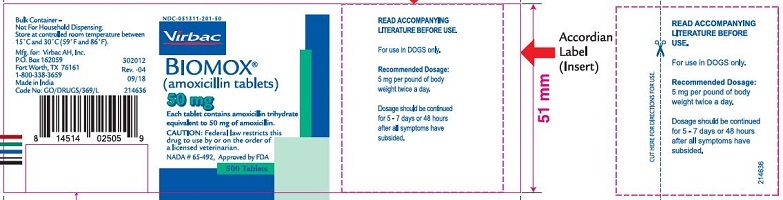
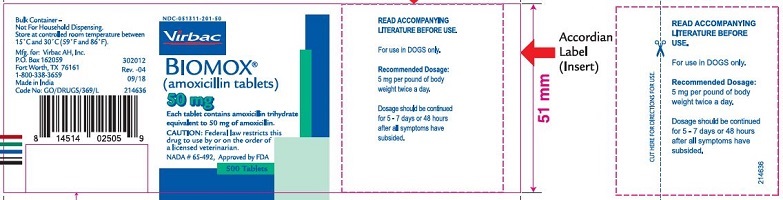
- PRINCIPAL DISPLAY PANEL
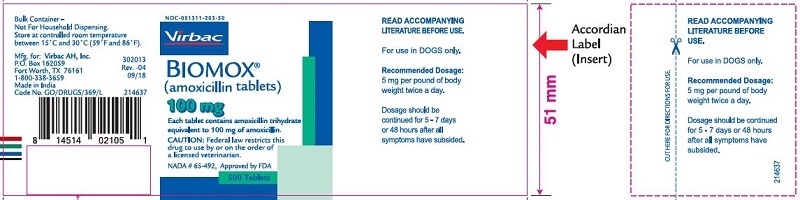
- PRINCIPAL DISPLAY PANEL
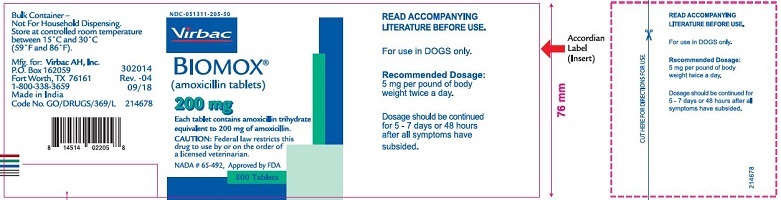
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOMOX
amoxicillin tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:51311-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 50 mg Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51311-201-50 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065492 05/24/2010 BIOMOX
amoxicillin tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:51311-203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 100 mg Product Characteristics Color white Score no score Shape ROUND Size 10mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51311-203-50 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065492 05/24/2010 BIOMOX
amoxicillin tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:51311-205 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amoxicillin (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 200 mg Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51311-205-50 500 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065492 05/24/2010 Labeler - Virbac AH, Inc. (131568396) Establishment Name Address ID/FEI Business Operations Medispray Laboratories Private Ltd 915793457 manufacture Establishment Name Address ID/FEI Business Operations Centrient Pharmaceuticals Netherlands 860184986 api manufacture Establishment Name Address ID/FEI Business Operations Alcami Missouri Corporation 117877975 analysis Establishment Name Address ID/FEI Business Operations Alcami Carolinas Corporation 831351445 analysis