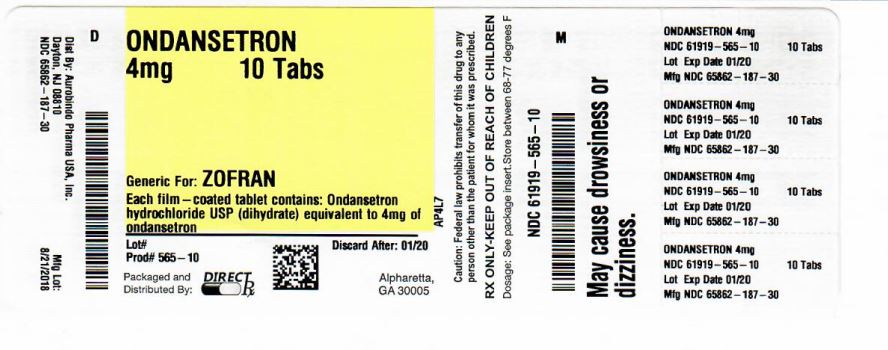
Label: ONDANSETRON tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 61919-565-10, 61919-565-20 - Packager: DIRECT RX
- This is a repackaged label.
- Source NDC Code(s): 65862-187
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 12, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
- Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy.
- Prevention of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy.
- Prevention of nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen.
- Prevention of postoperative nausea and/or vomiting. As with other antiemetics, routine prophylaxis is not recommended for patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and/or vomiting must be avoided postoperatively, ondansetron tablets, USP are recommended even where the incidence of postoperative nausea and/or vomiting is low.
- CONTRAINDICATIONS
- WARNINGS
- ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE AND ADMINISTRATION
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ONDANSETRON
ondansetron tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:61919-565(NDC:65862-187) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONDANSETRON HYDROCHLORIDE (UNII: NMH84OZK2B) (ONDANSETRON - UNII:4AF302ESOS) ONDANSETRON 4 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color white (WHITE TO OFF WHITE) Score no score Shape OVAL Size 6mm Flavor Imprint Code F;91 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61919-565-20 20 in 1 BOTTLE; Type 0: Not a Combination Product 03/23/2016 2 NDC:61919-565-10 1 in 1 BOTTLE; Type 0: Not a Combination Product 09/12/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078539 03/23/2016 Labeler - DIRECT RX (079254320) Establishment Name Address ID/FEI Business Operations DIRECT RX 079254320 repack(61919-565)