Label: BANAMINE- flunixin meglumine injection
- NDC Code(s): 0061-0851-03, 0061-0851-04
- Packager: Merck Sharp & Dohme Corp.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION50 mg/mL - Only for Intravenous Use in Beef and Dairy Cattle. Not for Use in Dry Dairy Cows and Veal Calves. For Intravenous and Intramuscular Use in Horses. PRODUCT - INFORMATION - Approved by ...
-
SPL UNCLASSIFIED SECTIONCAUTION Federal law restricts this drug to use by or on the order of a licensed veterinarian.
-
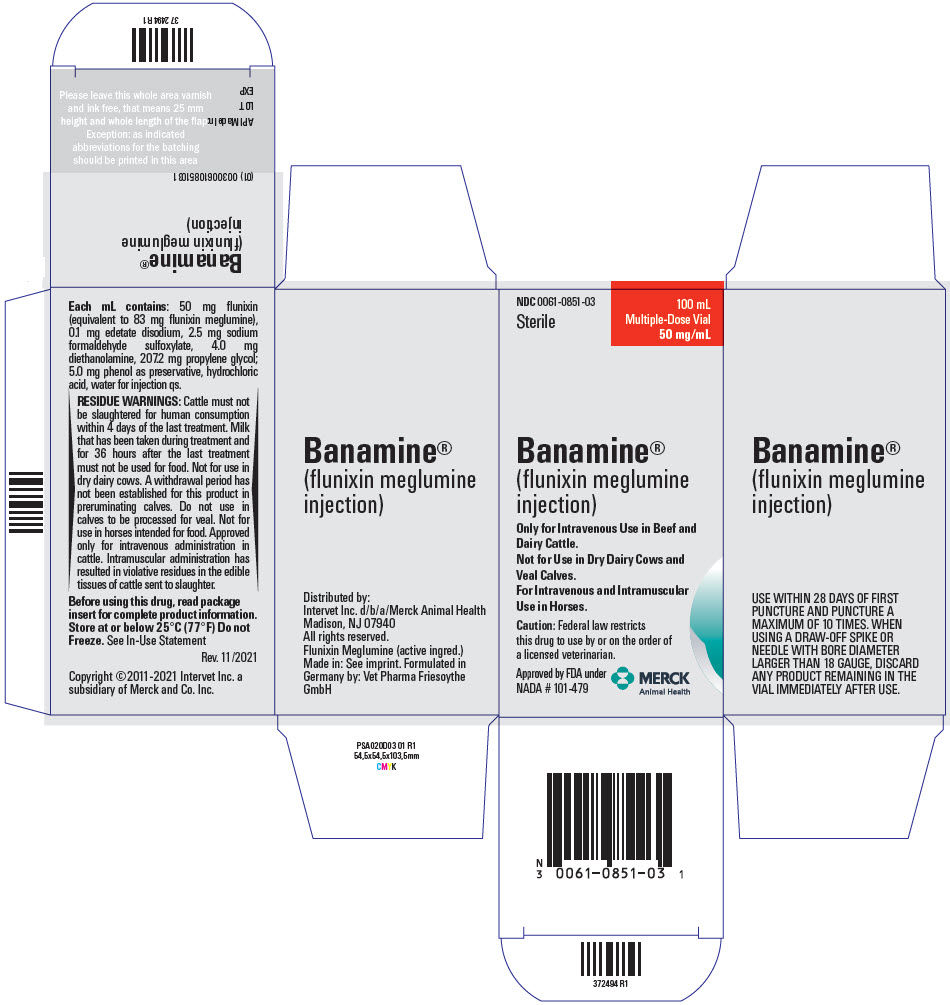
DESCRIPTIONDESCRIPTION Each milliliter of BANAMINE (flunixin meglumine injection) contains 50 mg flunixin (equivalent to 83 mg flunixin meglumine), 0.1 mg edetate disodium, 2.5 mg sodium formaldehyde ...
-
CLINICAL PHARMACOLOGYPHARMACOLOGY Flunixin meglumine is a potent, non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It is significantly more potent than pentazocine ...
-
VETERINARY INDICATIONSINDICATIONS Horse: BANAMINE (flunixin meglumine injection) is recommended for the alleviation of inflammation and pain associated with musculoskeletal disorders in the horse. It is also ...
-
DOSE AND ADMINISTRATIONUSE WITHIN 28 DAYS OF FIRST PUNCTURE AND PUNCTURE A MAXIMUM OF 10 TIMES. WHEN USING A DRAW-OFF SPIKE OR NEEDLE WITH BORE DIAMETER LARGER THAN 18 GAUGE, DISCARD ANY PRODUCT REMAINING IN THE VIAL ...
-
CONTRAINDICATIONSCONTRAINDICATIONS Horse: There are no known contraindications to this drug when used as directed. Intra-arterial injection should be avoided. Horses inadvertently injected intra-arterially can ...
-
RESIDUE WARNINGRESIDUE WARNINGS: Cattle must not be slaughtered for human consumption within 4 days of the last treatment. Milk that has been taken during treatment and for 36 hours after the last treatment must ...
-
PRECAUTIONSPRECAUTIONS As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal and renal toxicity. Sensitivity to drug-associated adverse effects varies with the individual ...
-
SPL UNCLASSIFIED SECTIONSAFETY Horse: A 3-fold intramuscular dose of 1.5 mg/lb of body weight daily for 10 consecutive days was safe. No changes were observed in hematology, serum chemistry, or urinalysis values ...
-
ADVERSE REACTIONSADVERSE REACTIONS In horses, isolated reports of local reactions following intramuscular injection, particularly in the neck, have been received. These include localized swelling, sweating ...
-
HOW SUPPLIEDHOW SUPPLIED BANAMINE (flunixin meglumine injection), 50 mg/ mL, is available in 100-mL (NDC 0061-0851-03), and 250-mL (NDC 0061-0851-04) multi-dose vials. Store at or below 25°C (77°F) Do not ...
-
REFERENCESREFERENCES - Johansson M, Anler EL. Gas chromatographic analysis of flunixin in equine urine after extractive methylation. J Chromatogr. 1988;427:55-66. Odensvik K, Johansson M. High-performance ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Intervet Inc d/b/a Merck Animal Health - Madison, NJ 07940 - Copyright©2011-2021 Intervet Inc., a subsidiary of Merck and - Co., Inc. All rights reserved. Formulated in Germany by: Vet ...
-
PRINCIPAL DISPLAY PANEL - 100 mL Vial CartonNDC 0061-0851-03 - Sterile - 100 mL - Multiple-Dose Vial - 50 mg/mL - Banamine® (flunixin meglumine - injection) Only for Intravenous Use in Beef and - Dairy Cattle. Not for Use in Dry Dairy Cows and - Veal ...
-
INGREDIENTS AND APPEARANCEProduct Information