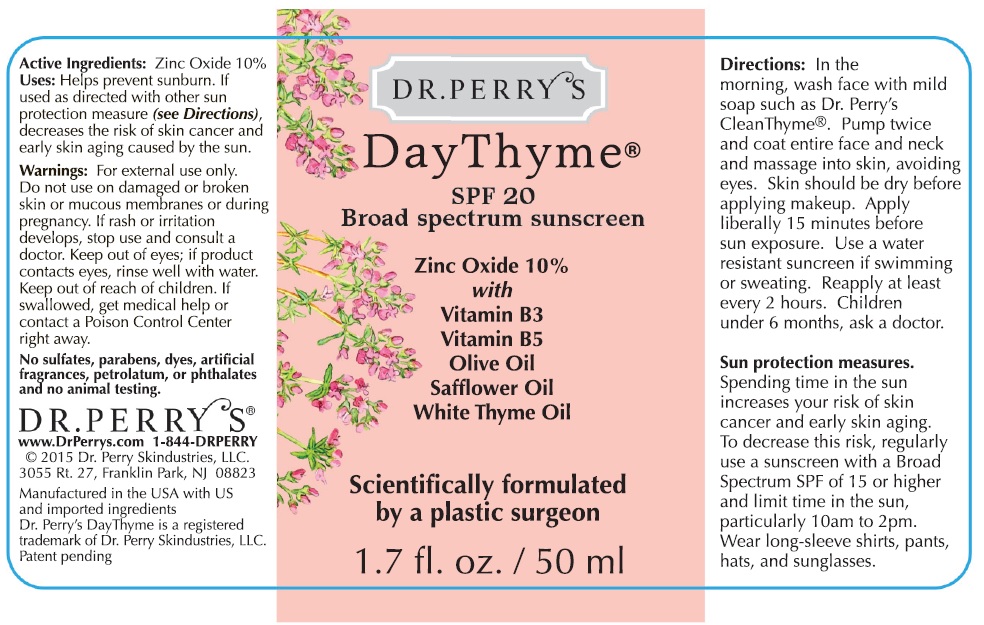
Label: DR. PERRYS DAY THYME SPF 20 BROAD SPECTRUM SUNSCREEN- zinc oxide gel
- NDC Code(s): 14268-017-00
- Packager: Englewood Lab, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
- In the morning, wash face with mild soap such as Dr. Perry's Clean Thyme. Pump twice and coat entire face and neck and massage into skin, avoiding eyes. Skin should be dry before applying makeup.
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, particularly between 10 am and 2 pm.
- wear long-sleeve shirts, pants, hats, and sunglasses.
- Children under 6 months: ask a doctor
-
Inacctive Ingredients:
Water, Glycerin, C12-15 Alkyl Benzoate, Pentylene Glycol, Niacinamide, Olea Europaea (Olive) Fruit Oil, Carthamus Tinctorius (Safflower) Seed Oil, Coconut Alkanes, Panthenol, Tocopherol, Citric Acid, Coco-Caprate Caprylate, Sodium Hyaluronate, Echium Plantagineum Seed Oil, Hydrogenated Lecithin, Thymus Vulgaris (Thyme) Flower/Leaf Oil, Isohexadecane, Glyceryl Isostearate, Polyhydroxystearic Acid, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Xanthan Gum, Phenoxyethanol, Benzyl Alcohol, Potassium Sorbate, Sodium Benzoate, Polysorbate 60
- Other information
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
DR. PERRYS DAY THYME SPF 20 BROAD SPECTRUM SUNSCREEN
zinc oxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14268-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PENTYLENE GLYCOL (UNII: 50C1307PZG) NIACINAMIDE (UNII: 25X51I8RD4) OLIVE OIL (UNII: 6UYK2W1W1E) SAFFLOWER OIL (UNII: 65UEH262IS) COCONUT ALKANES (UNII: 1E5KJY107T) PANTHENOL (UNII: WV9CM0O67Z) TOCOPHEROL (UNII: R0ZB2556P8) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ECHIUM PLANTAGINEUM SEED OIL (UNII: PIB7XBU8XW) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) THYME OIL (UNII: 2UK410MY6B) ISOHEXADECANE (UNII: 918X1OUF1E) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) XANTHAN GUM (UNII: TTV12P4NEE) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZYL ALCOHOL (UNII: LKG8494WBH) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) POLYSORBATE 60 (UNII: CAL22UVI4M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14268-017-00 1 in 1 CARTON 04/04/2016 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/04/2016 Labeler - Englewood Lab, Inc. (172198223) Establishment Name Address ID/FEI Business Operations Englewood Lab, Inc. 172198223 manufacture(14268-017)