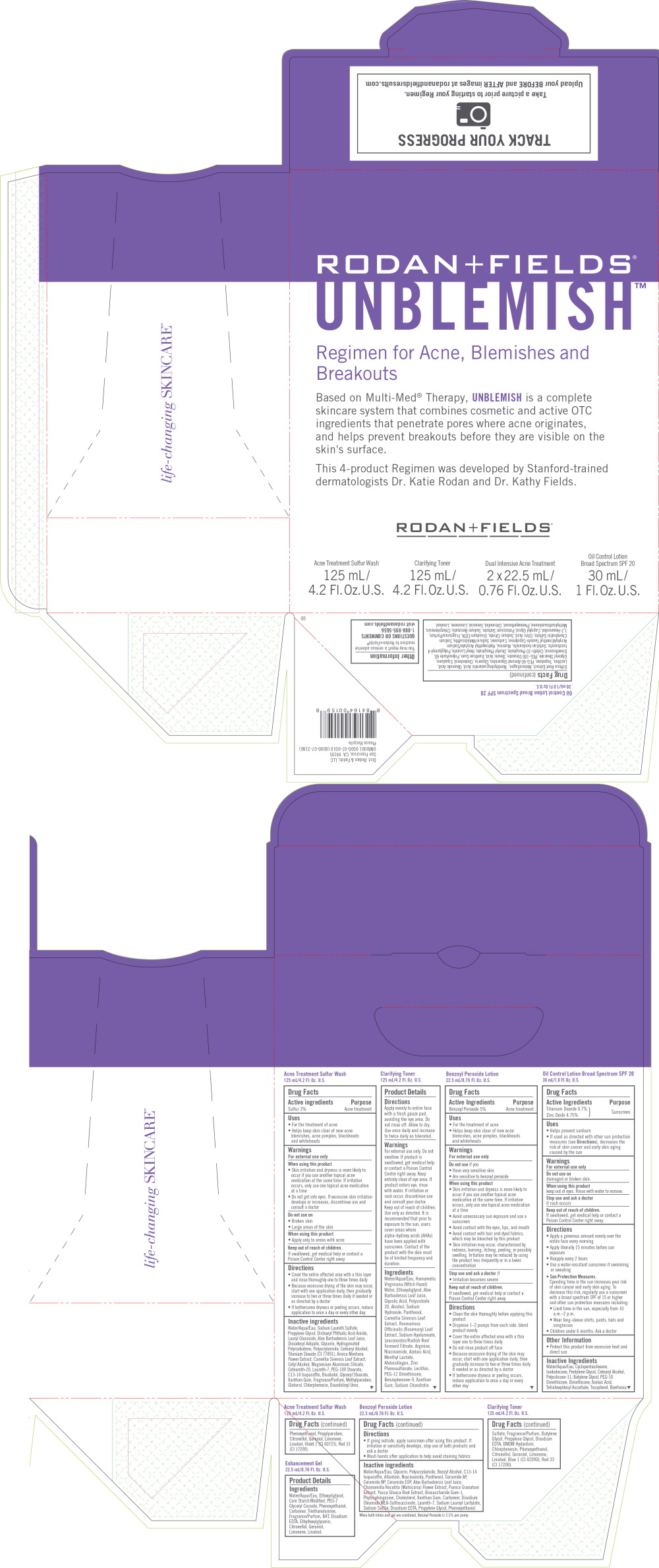
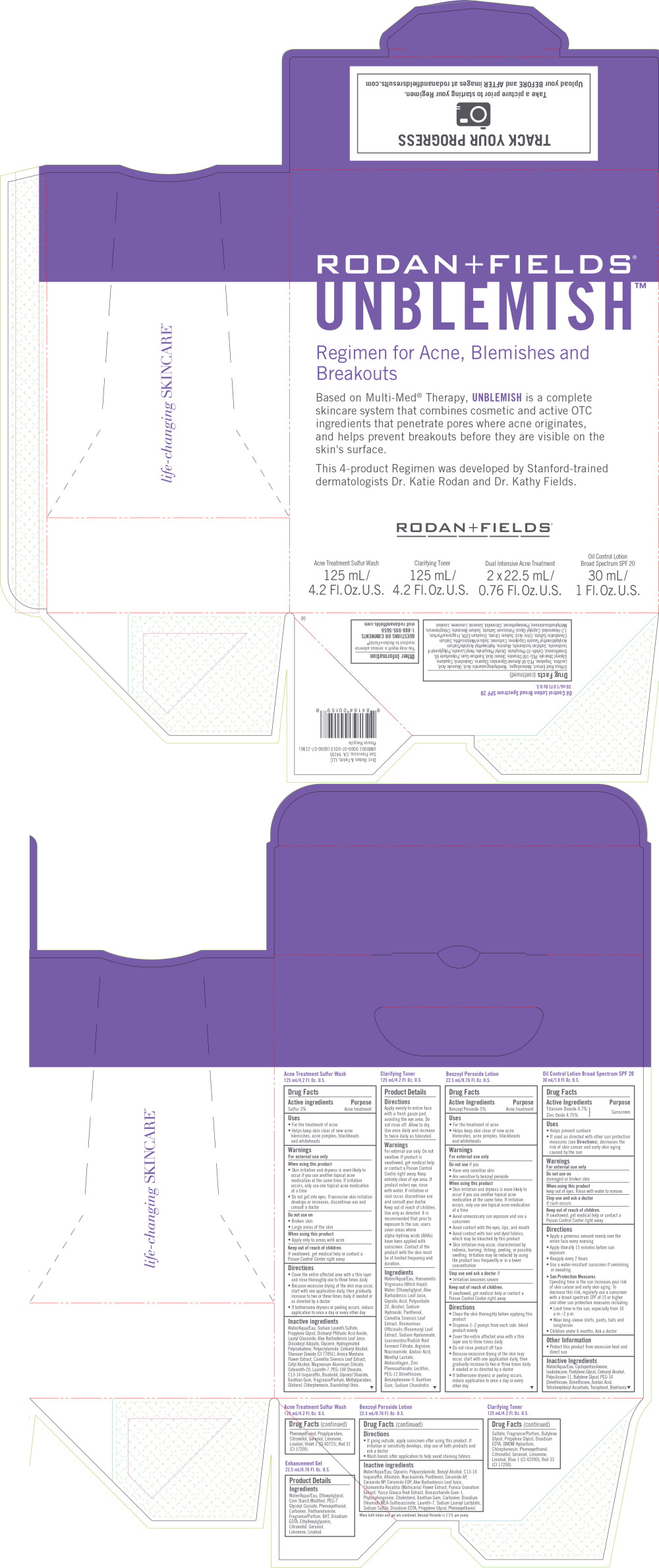
Label: RODAN FIELDS UNBLEMISH REGIMEN FOR ACNE, BLEMISHES AND BREAKOUTS- sulfur, benzoyl peroxide, titanium dioxide, zinc oxide kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 14222-2020-1 - Packager: Rodan & Fields, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 8, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Acne Treatment Sulfur Wash
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time
- do not get into eyes. If excessive skin irritation develops or increases, discontinue use and consult a doctor
-
Directions
- Cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
Inactive Ingredients
Water/Aqua/Eau, Sodium Laureth Sulfate, Propylene Glycol, Distearyl Phthalic Acid Amide, Lauryl Glucoside, Aloe Barbadensis Leaf Juice, Diisodecyl Adipate, Glycerin, Hydrogenated Polyisobutene, Polyacrylamide, Cetearyl Alcohol, Titanium Dioxide (CI 77891), Arnica Montana Flower Extract, Camellia Sinensis Leaf Extract, Cetyl Alcohol, Magnesium Aluminum Silicate, Ceteareth-20, Laureth-7, PEG-100 Stearate, C13-14 Isoparaffin, Bisabolol, Glyceryl Stearate, Xanthan Gum, Fragrance/Parfum, Methylparaben, Glutaral, Chlorphenesin, Diazolidinyl Urea, Phenoxyethanol, Propylparaben, Citronellol, Geraniol, Limonene, Linalool, Violet 2 (CI 60725), Red 33 (CI 17200).
- Questions
- Clarifying Toner
-
Warnings
For external use only. Do not swallow. If product is swallowed, get medical help or contact a Poison Control Centre right away. Keep entirely clear of eye area. If product enters eye, rinse with water. If irritation or rash occur, discontinue use and consult your doctor. Keep out of reach of children. Use only as directed. It is recommended that prior to exposure to the sun, users cover areas where alpha-hydroxy acids (AHAs) have been applied with sunscreen. Contact of the product with the skin must be of limited frequency and duration.
-
Ingredients
Water/Aqua/Eau, Hamamelis Virginiana (Witch Hazel) Water, Ethoxydiglycol, Aloe Barbadensis Leaf Juice, Glycolic Acid, Polysorbate 20, Alcohol, Sodium Hydroxide, Panthenol, Camellia Sinensis Leaf Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Sodium Hyaluronate, Leuconostoc/Radish Root Ferment Filtrate, Arginine, Niacinamide, Azelaic Acid, Menthyl Lactate, Atelocollagen, Zinc Phenosulfonate, Lecithin, PEG-12 Dimethicone, Benzophenone-9, Xanthan Gum, Sodium Chondrotin Sulfate, Fragrance/Parfum, Butylene Glycol, Propylene Glycol, Disodium EDTA, DMDM Hydantoin, Chlorphenesin, Phenoxyethanol, Citronellol, Geraniol, Limonene, Linalool, Blue 1 (CI 42090), Red 33 (CI 17200).
- Dual Intensive Acne Treatment
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips, and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- Clean the skin thoroughly before applying this product
- Dispense 1–2 pumps from each side. Blend product evenly
- Cover the entire affected area with a thin layer one to three times daily
- Do not rinse product off face
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor
- Wash hands after application to help avoid staining fabrics
-
Inactive Ingredients
Water/Aqua/Eau, Ethoxydiglycol, Glycerin, Polyacrylamide, Allantoin, Niacinamide, Panthenol, Ceramide AP, Ceramide NP, Ceramide EOP, Punica Granatum Extract, Yucca Glauca Root Extract, Chamomilla Recutita (Matricaria) Flower Extract, Aloe Barbadensis Leaf Juice, Biosaccharide Gum-1, Phytosphingosine, Cholesterol, Corn Starch Modified, C13-14 Isoparaffin, PEG-7 Glyceryl Cocoate, Disodium Oleamido MEA-Sulfosuccinate, Laureth-7, Sodium Lauroyl Lactylate, Sodium Sulfite, Xanthan Gum, Carbomer, Triethanolamine, Fragrance/Parfum, Disodium EDTA, BHT, Propylene Glycol, Ethylhexylglycerin, Phenoxyethanol, Benzyl Alcohol, Citronellol, Geraniol, Limonene, Linalool.
- Questions
- Oil Control Lotion Broad Spectrum SPF 20
- Active Ingredients
- Purpose
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- Apply a generous amount evenly over the entire face every morning
- Apply liberally 15 minutes before sun exposure
- Reapply every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
-
Sun Protection Measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- Children under 6 months: Ask a doctor
- Other Information
-
Inactive Ingredients
Water/Aqua/Eau, Cyclopentasiloxane, Isododecane, Pentylene Glycol, Cetearyl Alcohol, Polysilicone-11, Butylene Glycol, PEG-10 Dimethicone, Dimethicone, Azelaic Acid, Tetrahexyldecyl Ascorbate, Tocopherol, Boerhavia Diffusa Root Extract, Atelocollagen, Nordihydroguaiaretic Acid, Oleanolic Acid, Lecithin, Tropolone, PEG-60 Almond Glycerides, Glycerin, Cholesterol, Squalane, Glyceryl Stearate, PEG-100 Stearate, Stearic Acid, Xanthan Gum, Polysorbate 60, Dimethiconol, Ceteth-10 Phosphate, Dicetyl Phosphate, Hexyl Laurate, Polyglyceryl-4 Isostearate, Sorbitan Isostearate, Alumina, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Carbomer, Sodium Metabisulfite, Sodium Chondroitin Sulfate, Citric Acid, Sodium Citrate, Disodium EDTA, Fragrance/Parfum, 1,2-Hexanediol, Caprylyl Glycol, Potassium Sorbate, Sodium Benzoate, Chlorphenesin, Methylisothiazolinone, Phenoxyethanol, Citronellol, Geraniol, Limonene, Linalool.
- Questions
- Principal display Panel - Rodan Fields Unblemish Acne Treatment Sulfur Wash Label
- Principal display Panel - Rodan Fields Unblemish Dual Intensive Acne Treatment Label
- Principal display Panel - Rodan Fields Unblemish Oil Control Lotion Label
-
Principal display Panel - Rodan Fields Unblemish Kit Label
RODAN + FIELDS®
UNBLEMISH™Regiment for Acne, Blemishes and Breakouts
Based on Multi-Med® Therapy, UNBLEMISH is a complete
skincare system that combines cosmetic and active OTC
ingredients that penetrate pores where acne originates,
and helps prevent breakouts before they are visible on the
skin's surface.This 4-product Regimen was developed by Stanford-trained
dermatologists Dr. Katie Rodan and Dr. Kathy Fields.RODAN + FIELDS®
Acne Treatment Sulfur Wash
125 mL/
4.2 Fl.Oz. U.S.Clarifying Toner
125 mL/
4.2 Fl.Oz. U.S.Dual Intensive Acne Treatment
2 x 22.5 mL/
0.76 Fl.Oz. U.S.Oil Control Lotion
Broad Spectrum SPF 2030 mL/
1 Fl.Oz. U.S. -
INGREDIENTS AND APPEARANCE
RODAN FIELDS UNBLEMISH REGIMEN FOR ACNE, BLEMISHES AND BREAKOUTS
sulfur, benzoyl peroxide, titanium dioxide, zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-2020 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-2020-1 1 in 1 CARTON; Type 0: Not a Combination Product 06/01/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 125 mL Part 2 2 BOTTLE, PUMP 45 mL Part 3 1 TUBE 30 mL Part 1 of 3 UNBLEMISH ACNE TREATMENT SULFUR WASH
sulfur creamProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulfur (UNII: 70FD1KFU70) (Sulfur - UNII:70FD1KFU70) Sulfur 3.0 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Sodium Laureth Sulfate (UNII: BPV390UAP0) Propylene Glycol (UNII: 6DC9Q167V3) Distearyl Phthalamic Acid (UNII: 5552GSZ9LI) Lauryl Glucoside (UNII: 76LN7P7UCU) Aloe Vera Leaf (UNII: ZY81Z83H0X) Diisodecyl Adipate (UNII: 3V0Q382O0P) Glycerin (UNII: PDC6A3C0OX) Hydrogenated Polybutene (1300 MW) (UNII: 7D1YQ9Y5EZ) Polyacrylamide (1500 MW) (UNII: 5D6TC4BRWV) Cetostearyl Alcohol (UNII: 2DMT128M1S) Titanium Dioxide (UNII: 15FIX9V2JP) Arnica Montana Flower (UNII: OZ0E5Y15PZ) Green Tea Leaf (UNII: W2ZU1RY8B0) Cetyl Alcohol (UNII: 936JST6JCN) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Laureth-7 (UNII: Z95S6G8201) PEG-100 Stearate (UNII: YD01N1999R) C13-14 Isoparaffin (UNII: E4F12ROE70) Levomenol (UNII: 24WE03BX2T) Glyceryl Monostearate (UNII: 230OU9XXE4) Xanthan Gum (UNII: TTV12P4NEE) Methylparaben (UNII: A2I8C7HI9T) Glutaral (UNII: T3C89M417N) Chlorphenesin (UNII: I670DAL4SZ) Diazolidinyl Urea (UNII: H5RIZ3MPW4) Phenoxyethanol (UNII: HIE492ZZ3T) Propylparaben (UNII: Z8IX2SC1OH) .Beta.-Citronellol, (R)- (UNII: P01OUT964K) Geraniol (UNII: L837108USY) Limonene, (+)- (UNII: GFD7C86Q1W) Linalool, (+/-)- (UNII: D81QY6I88E) D&C Violet No. 2 (UNII: 350KA7O6HK) D&C Red No. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 125 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/01/2017 Part 2 of 3 UNBLEMISH BENZOYL PEROXIDE
benzoyl peroxide lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzoyl Peroxide (UNII: W9WZN9A0GM) (Benzoyl Peroxide - UNII:W9WZN9A0GM) Benzoyl Peroxide 5.0 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Diethylene Glycol Monoethyl Ether (UNII: A1A1I8X02B) Glycerin (UNII: PDC6A3C0OX) Polyacrylamide (1500 MW) (UNII: 5D6TC4BRWV) Allantoin (UNII: 344S277G0Z) Niacinamide (UNII: 25X51I8RD4) Panthenol (UNII: WV9CM0O67Z) Ceramide AP (UNII: F1X8L2B00J) Ceramide NP (UNII: 4370DF050B) Ceramide 1 (UNII: 5THT33P7X7) Punica Granatum Root Bark (UNII: CLV24I3T1D) Yucca Glauca Root (UNII: 1A15YBH7N1) Chamomile (UNII: FGL3685T2X) Aloe Vera Leaf (UNII: ZY81Z83H0X) Biosaccharide Gum-1 (UNII: BB4PU4V09H) Phytosphingosine (UNII: GIN46U9Q2Q) Cholesterol (UNII: 97C5T2UQ7J) Modified Corn Starch (1-Octenyl Succinic Anhydride) (UNII: 461P5CJN6T) C13-14 Isoparaffin (UNII: E4F12ROE70) PEG-7 Glyceryl Cocoate (UNII: VNX7251543) Disodium Oleamido MEA-Sulfosuccinate (UNII: 5M1101WGSY) Laureth-7 (UNII: Z95S6G8201) Sodium Lauroyl Lactylate (UNII: 7243K85WFO) Sodium Sulfite (UNII: VTK01UQK3G) Xanthan Gum (UNII: TTV12P4NEE) Carboxypolymethylene (UNII: 0A5MM307FC) Trolamine (UNII: 9O3K93S3TK) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Butylated Hydroxytoluene (UNII: 1P9D0Z171K) Propylene Glycol (UNII: 6DC9Q167V3) Ethylhexylglycerin (UNII: 147D247K3P) Phenoxyethanol (UNII: HIE492ZZ3T) Benzyl Alcohol (UNII: LKG8494WBH) .Beta.-Citronellol, (R)- (UNII: P01OUT964K) Geraniol (UNII: L837108USY) Limonene, (+)- (UNII: GFD7C86Q1W) Linalool, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 22.5 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/01/2017 Part 3 of 3 UNBLEMISH OIL CONTROL BROAD SPECTRUM SPF 20
titanium dioxide, zinc oxide lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 0.7 g in 100 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 4.75 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Cyclomethicone 5 (UNII: 0THT5PCI0R) Isododecane (UNII: A8289P68Y2) Pentylene Glycol (UNII: 50C1307PZG) Cetostearyl Alcohol (UNII: 2DMT128M1S) Dimethicone/Vinyl Dimethicone Crosspolymer (Soft Particle) (UNII: 9E4CO0W6C5) Butylene Glycol (UNII: 3XUS85K0RA) PEG-10 Dimethicone (600 CST) (UNII: 8PR7V1SVM0) Dimethicone (UNII: 92RU3N3Y1O) Azelaic Acid (UNII: F2VW3D43YT) Tetrahexyldecyl Ascorbate (UNII: 9LBV3F07AZ) Tocopherol (UNII: R0ZB2556P8) Boerhavia Diffusa Root (UNII: KR0SR09KYL) Marine Collagen, Soluble (UNII: 8JC99XGU4W) Nordihydroguaiaretic Acid, (+/-)- (UNII: 7PZ73W4ZNR) Oleanolic Acid (UNII: 6SMK8R7TGJ) Lecithin, Soybean (UNII: 1DI56QDM62) Tropolone (UNII: 7L6DL16P1T) PEG-60 Almond Glycerides (UNII: 4Y0E651N0F) Glycerin (UNII: PDC6A3C0OX) Cholesterol (UNII: 97C5T2UQ7J) Squalane (UNII: GW89575KF9) Glyceryl Monostearate (UNII: 230OU9XXE4) PEG-100 Stearate (UNII: YD01N1999R) Stearic Acid (UNII: 4ELV7Z65AP) Xanthan Gum (UNII: TTV12P4NEE) Polysorbate 60 (UNII: CAL22UVI4M) Dimethiconol (70 CST) (UNII: MOT8IL21AR) Ceteth-10 Phosphate (UNII: 4E05O5N49G) Dihexadecyl Phosphate (UNII: 2V6E5WN99N) Hexyl Laurate (UNII: 4CG9F9W01Q) Polyglyceryl-4 Isostearate (UNII: 820DPX33S7) Sorbitan Isostearate (UNII: 01S2G2C1E4) Aluminum Oxide (UNII: LMI26O6933) Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer (100000 MPA.S At 1.5%) (UNII: 86FQE96TZ4) Carboxypolymethylene (UNII: 0A5MM307FC) Sodium Metabisulfite (UNII: 4VON5FNS3C) Chondroitin Sulfate Sodium (Bovine) (UNII: 8QTV3DTT8W) Citric Acid Monohydrate (UNII: 2968PHW8QP) Sodium Citrate (UNII: 1Q73Q2JULR) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) 1,2-Hexanediol (UNII: TR046Y3K1G) Caprylyl Glycol (UNII: 00YIU5438U) Potassium Sorbate (UNII: 1VPU26JZZ4) Sodium Benzoate (UNII: OJ245FE5EU) Chlorphenesin (UNII: I670DAL4SZ) Methylisothiazolinone (UNII: 229D0E1QFA) Phenoxyethanol (UNII: HIE492ZZ3T) .Beta.-Citronellol, (R)- (UNII: P01OUT964K) Geraniol (UNII: L837108USY) Limonene, (+)- (UNII: GFD7C86Q1W) Linalool, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/01/2017 Labeler - Rodan & Fields, LLC. (051659584)