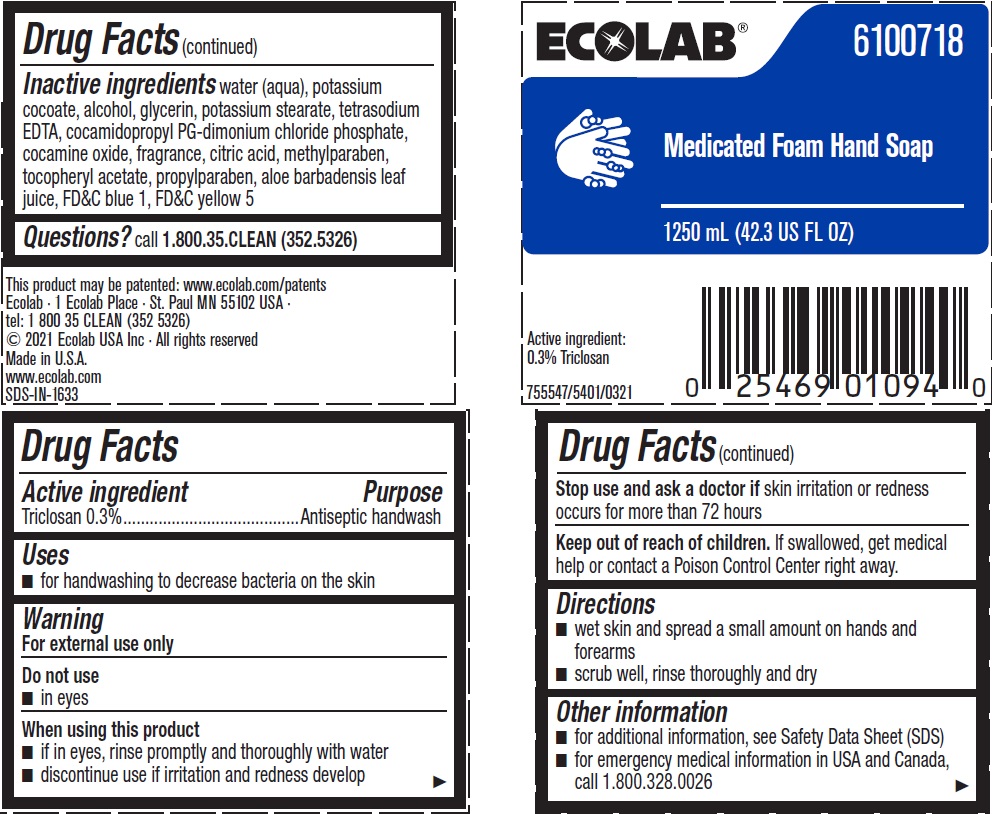
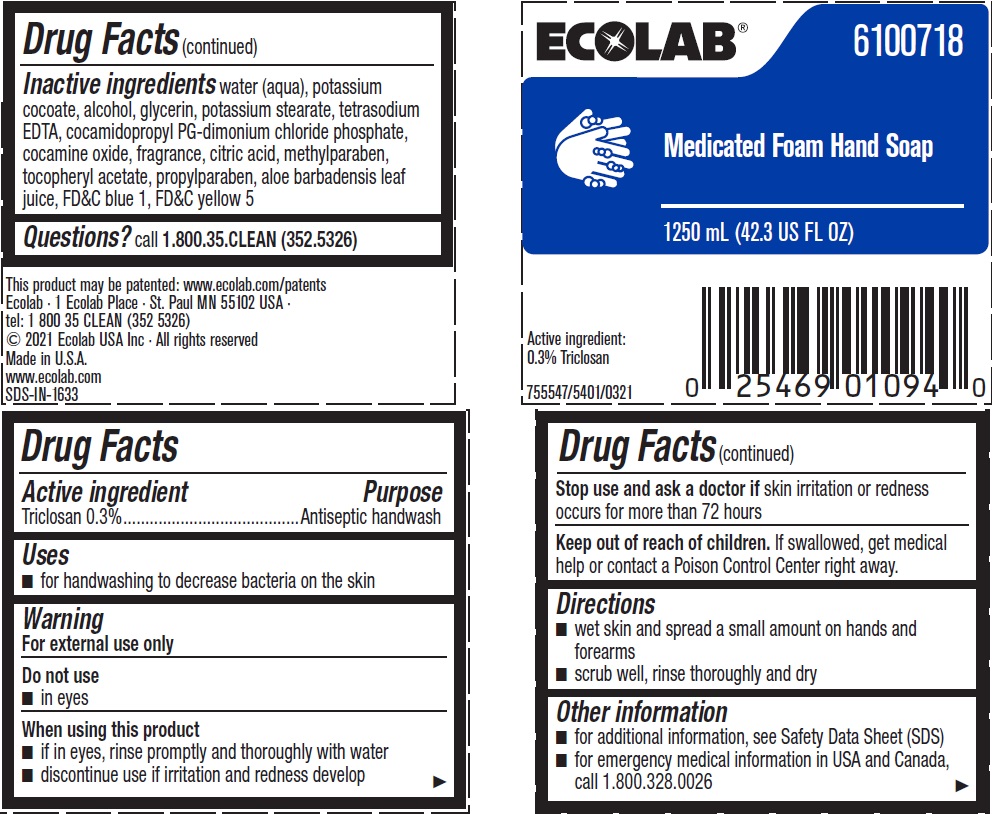
Label: ECOLAB- triclosan solution
- NDC Code(s): 47593-469-41, 47593-469-56, 47593-469-59
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
INACTIVE INGREDIENT
Inactive ingredients water (aqua), potassium cocoate, alcohol, glycerin, potassium stearate, tetrasodium EDTA, cocamidopropyl PG-dimonium chloride phosphate, cocamine oxide, fragrance, citric acid, methylparaben, tocopheryl acetate, propylparaben, aloe barbadensis leaf juice, FD&C blue 1, FD&C yellow 5
- QUESTIONS
- Principal display panel and representative label
-
INGREDIENTS AND APPEARANCE
ECOLAB
triclosan solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-469 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRICLOSAN (UNII: 4NM5039Y5X) (TRICLOSAN - UNII:4NM5039Y5X) TRICLOSAN 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM COCOATE (UNII: F8U72V8ZXP) ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM STEARATE (UNII: 17V812XK50) EDETATE SODIUM (UNII: MP1J8420LU) COCAMIDOPROPYL PG-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) COCAMINE OXIDE (UNII: QWA2IZI6FI) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) METHYLPARABEN (UNII: A2I8C7HI9T) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-469-56 1200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/24/2011 06/08/2023 2 NDC:47593-469-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/17/2013 3 NDC:47593-469-59 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/17/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/24/2011 Labeler - Ecolab Inc. (006154611)