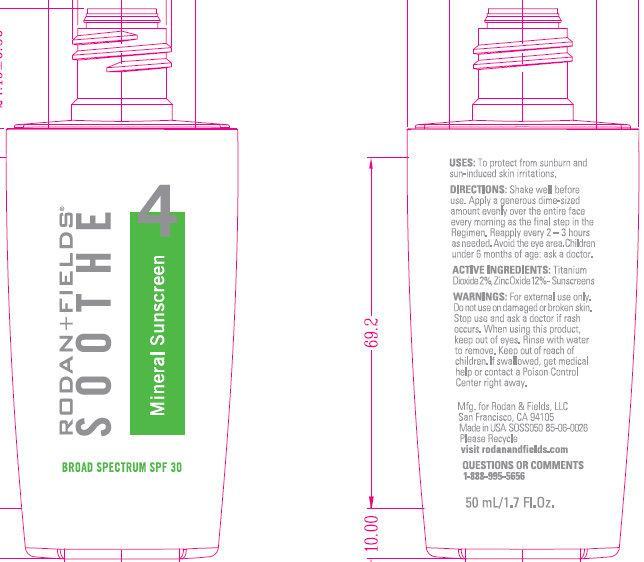
Label: RODAN FIELDS SOOTHE 4 MINERAL SUNSCREEN BROAD SPECTRUM SPF 30- titanium dioxide cream
- NDC Code(s): 14222-0000-1
- Packager: Rodan & Fields
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- RODAN + FIELDS SOOTHE 4 Mineral Sunscreen BROAD SPECTRUM SPF 30
- ACTIVE INGREDIENTS:
- PURPOSE
- USES:
- WARNINGS:
- DIRECTIONS:
-
INACTIVE INGREDIENTS:
Alumina, Aluminum Starch Octenylsuccinate, Butylene Glycol, Camellia Sinesis Extract, Caprylyl Glycol, Caprylyl Methicone, Cyclopentasiloxane, Dimethicone, Dimethicone/Vinyl Dimethicone Crosspolymer, Dipotassium Glycyrrhizate, Disodium EDTA, Disteardimonium Hectorite, Ethylhexylglycerin, Glycerin, Glycyrrhiza Glabra (Licorice) Root Extract, Hydrolyzed Wheat Protein/PVP Crosspolymer, Methicone, Panthenol, PEG-10 Dimethicone, Phenoxyethanol, Silica, Sodium Chloride, Sodium Hyaluronate, Sodium PCA, Tocopheryl Acetate, Water
- OTHER INFORMATION
- RODAN + FIELDS SOOTHE 4 Mineral Sunscreen BROAD SPECTRUM SPF 30 50ml (14222-0000-1)
-
INGREDIENTS AND APPEARANCE
RODAN FIELDS SOOTHE 4 MINERAL SUNSCREEN BROAD SPECTRUM SPF 30
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14222-0000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 20 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALUMINUM OXIDE (UNII: LMI26O6933) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) CAMELLIA SINENSIS FLOWER (UNII: 9I2BJY2J17) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) EDETATE DISODIUM (UNII: 7FLD91C86K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) LICORICE (UNII: 61ZBX54883) HYDROLYZED WHEAT PROTEIN (ENZYMATIC, 3000 MW) (UNII: J2S07SB0YL) PANTHENOL (UNII: WV9CM0O67Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14222-0000-1 1 in 1 CARTON 12/19/2017 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/03/2013 Labeler - Rodan & Fields (051659584) Establishment Name Address ID/FEI Business Operations Englewood Lab, Inc. 172198223 manufacture(14222-0000)