Label: CORID TYPE A MEDICATED ARTICLE- amprolium granule
- NDC Code(s): 23243-9704-1
- Packager: Huvepharma, Inc
- Category: OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated December 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- VETERINARY INDICATIONS
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- INDICATIONS & USAGE
- RESIDUE WARNING
- USER SAFETY WARNINGS
-
PRECAUTIONS
CAUTION: For satisfactory diagnosis, a microscopic examination of the feces should be done by a veterinarian or
diagnostic laboratory before treatment. When treating outbreaks, drug should be administered promptly after
diagnosis is determined.Do not use in feeds containing bentonite.
Use as the sole source of amprolium.
Restricted Drug (California) – Use only as directed. -
DOSAGE & ADMINISTRATION
MIXING DIRECTIONS: CORID 25% (amprolium) Type A medicated article should be thoroughly and evenly mixed
in the feed. CORID 25% may be used to manufacture a Type B medicated feed with a concentration of 2.5%
amprolium or a Type C medicated feed in the concentration range of 0.0125% to 1.25% amprolium.
• Use 200 pounds of CORID 25% per ton of feed to produce
Type B medicated feed containing 2.5% amprolium.
• Use 1 pound of CORID 25% per ton of feed to produce
Type C medicated feed containing 0.0125% amprolium.*
• Use 100 pounds of CORID 25% per ton of feed to produce
Type C medicated feed containing 1.25% amprolium.
• Use 10 pounds of 2.5% Type B per ton of feed to produce
Type C medicated feed containing 0.0125% amprolium.
• Use 1000 pounds of 2.5% Type B per ton of feed to produce
Type C medicated feed containing 1.25% amprolium.AMOUNT AND DIRECTIONS FOR USE:
Prevention: 227 mg amprolium /100 lb (5 mg/kg) body weight per day for 21 days during periods of exposure or when
experience indicates that coccidiosis is likely to be a hazard.
Treatment: 454 mg amprolium/100 lb (10 mg/kg) bodyweight per day for 5 days. Use on a herd basis only; when one
or more calves show signs of coccidiosis, it is likely that the rest of the group has been exposed and all
calves in the group should be treated.
*To aid in even distribution of CORID 25% in the finished feed, prepare a mixture of CORID 25% in a portion of complete feed
ingredient before mixing into the finished ration. Blend the mixture with the remainder of the finished feed and mix thoroughly.The proper amount of amprolium supplement is mixed with the amount of ration consumed in one day. The tables below
give suggested examples for using Type C medicated feed.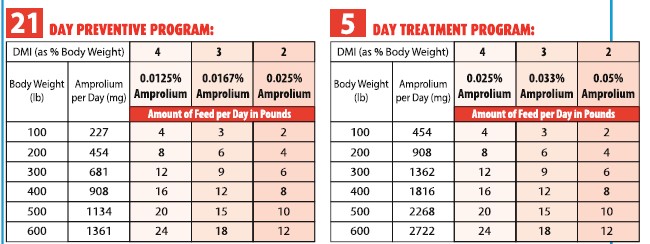
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CORID TYPE A MEDICATED ARTICLE
amprolium granuleProduct Information Product Type OTC TYPE A MEDICATED ARTICLE ANIMAL DRUG Item Code (Source) NDC:23243-9704 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength amprolium (UNII: 95CO6N199Q) (AMPROLIUM ION - UNII:H2T307KMZR) amprolium 250 mg in 0.001 kg Inactive Ingredients Ingredient Name Strength AMINO ACIDS, CORN GLUTEN (UNII: 0540V8ZD7V) SOYBEAN OIL (UNII: 241ATL177A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23243-9704-1 22.68 kg in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA012350 11/30/2017 Labeler - Huvepharma, Inc (619153559) Registrant - Huvepharma EOOD (552691651) Establishment Name Address ID/FEI Business Operations Huvepharma, Inc. 883128204 medicated animal feed manufacture, analysis, pack, label, manufacture



