Label: LAURA MERCIER FOUNDATION PRIMER PROTECT SPF 30- avobenzone, octinoxate, octocrylene, oxybenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 65342-7001-5, 65342-7003-0, 65342-7005-0 - Packager: Gurwitch Products, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 15, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
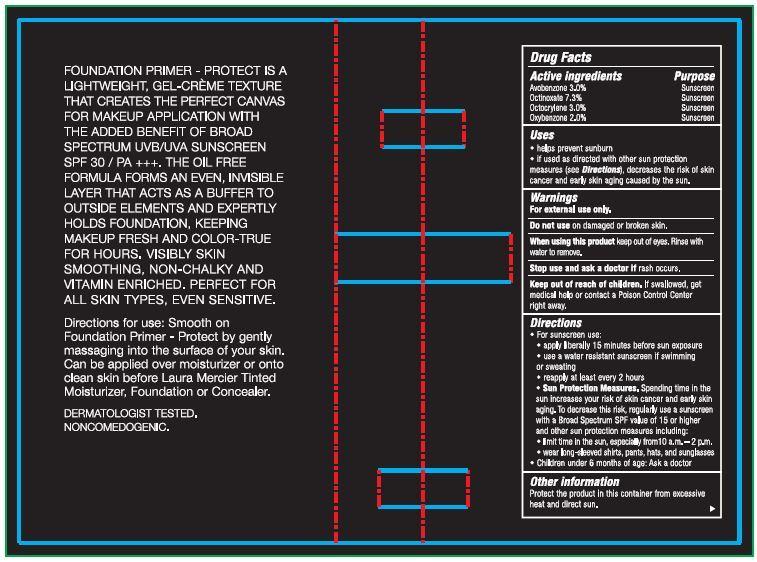
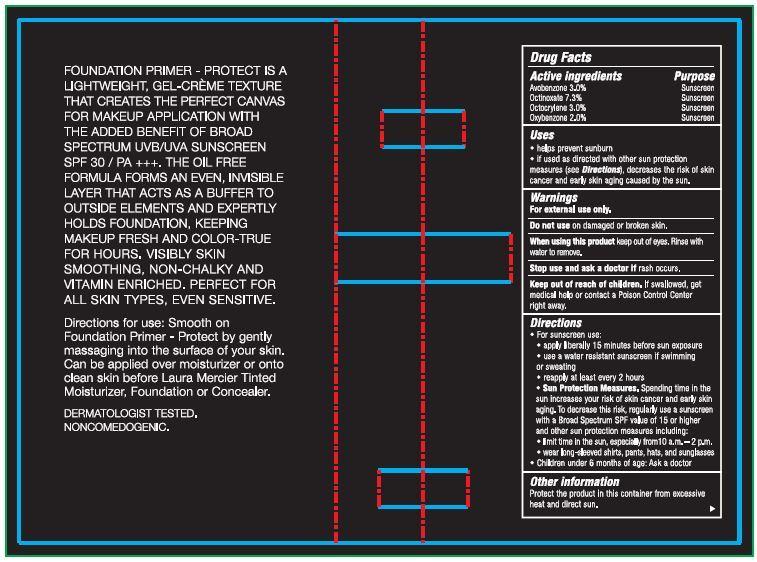
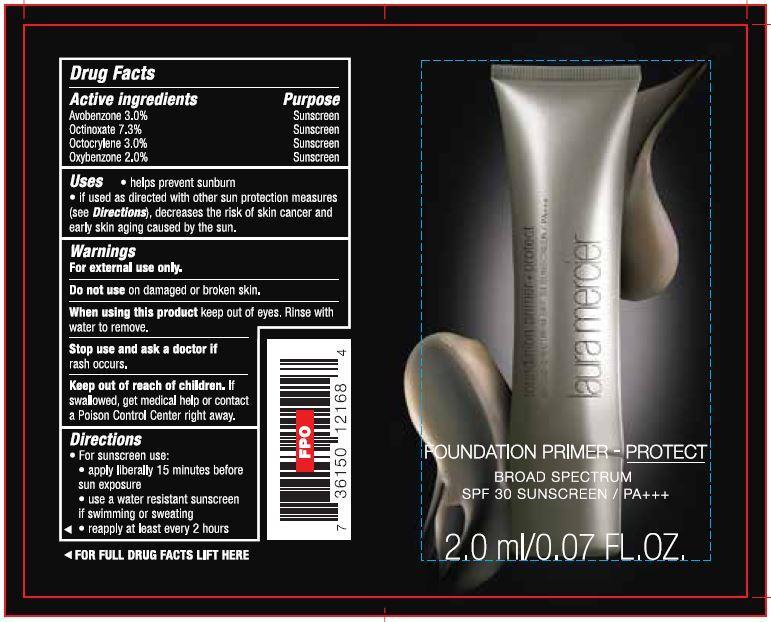
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
- For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least ever y 2 hours
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection mea sures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
FOUNDATION PRIMER PROTECT
FOUNDATION PRIMER - PROTECT IS A LIGHTWEIGHT, GEL-CREME TEXTURE THAT CREATES THE PERFECT CANVAS FOR MAKEUP APPLICATION WITH THE ADDED BENEFIT OF UVB/UVA BROAD SPECTRUM SPF 30 SUNSCREEN / PA +++. THE OIL FREE FORMULA FORMS AN EVEN, INVISIBLE LAYER THAT ACTS AS A BUFFER TO OUTSIDE ELEMENTS AND EXPERTLY HOLDS FOUNDATION, KEEPING MAKEUP FRESH AND COLOR-TRUE FOR HOURS. VISIBLY SKIN SMOOTHING, NON-CHALKY AND VITAMIN ENRICHED. PERFECT FOR ALL SKIN TYPES, EVEN SENSITIVE.
laura mercier DIST. GURWITCH PRODUCTS, L.L.C. PO BOX 7568, NEW YORK, NY 10150 MADE IN USA lauramercier.com 001 FOUNDATION PRIMER - PROTECT FPO 7 36150 11726 7
Directions for use: Smooth on Foundation Primer - Protect by gently massaging into the surface of your skin. Can be applied over moisturizer or onto clean skin before Laura Mercier Tinted Moisturizer, Foundation or Concealer. DERMATOLOGIST TESTED. NONCOMEDOGENIC.
FOUNDATION PRIMER - PROTECT FPO 7 36150 12021 2 002
Directions for use: Smooth on Foundation Primer - Protect by gently massaging into the surface of your skin. Can be applied over moisturizer or onto clean skin before Laura Mercier Tinted Moisturizer, Foundation or Concealer.
DERMATOLOGIST TESTED. NONCOMEDOGENIC.
Warnings: For external use only. Avoid contact with eyes. Discontinue use if signs of rash or irritation appear. If irritation persists, consult a doctor.
- PRINCIPAL DISPLAY PANEL
- Foundation Primer Broad Spectrum SPF 30 1.7 FL.OZ./50 ml
- Foundation Primer Broad Spectrum SPF 30 0.5 FL. OZ. / 15 ml
- Foundation Primer Broad Spectrum SPF 30 2.0ml/0.07 FL.OZ.
-
INGREDIENTS AND APPEARANCE
LAURA MERCIER FOUNDATION PRIMER PROTECT SPF 30
avobenzone, octinoxate, octocrylene, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65342-7005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 73 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 30 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISOHEXADECANE (UNII: 918X1OUF1E) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TOCOPHEROL (UNII: R0ZB2556P8) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ASCORBIC ACID (UNII: PQ6CK8PD0R) D&C RED NO. 6 (UNII: 481744AI4O) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65342-7005-0 1 in 1 CARTON 1 50 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/11/2014 LAURA MERCIER FOUNDATION PRIMER PROTECT SPF 30
avobenzone, octinoxate, octocrylene, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65342-7003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 73 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 30 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISOHEXADECANE (UNII: 918X1OUF1E) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TOCOPHEROL (UNII: R0ZB2556P8) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ASCORBIC ACID (UNII: PQ6CK8PD0R) D&C RED NO. 6 (UNII: 481744AI4O) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65342-7003-0 1 in 1 CARTON 1 15 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/11/2014 LAURA MERCIER FOUNDATION PRIMER PROTECT SPF 30
avobenzone, octinoxate, octocrylene, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65342-7001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 73 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 30 mg in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISOHEXADECANE (UNII: 918X1OUF1E) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TOCOPHEROL (UNII: R0ZB2556P8) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) ASCORBYL PALMITATE (UNII: QN83US2B0N) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ASCORBIC ACID (UNII: PQ6CK8PD0R) D&C RED NO. 6 (UNII: 481744AI4O) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65342-7001-5 1 in 1 CARTON 1 2 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/11/2014 Labeler - Gurwitch Products, LLC (884875246) Establishment Name Address ID/FEI Business Operations Oxygen Development LLC 137098492 manufacture(65342-7005, 65342-7003, 65342-7001)