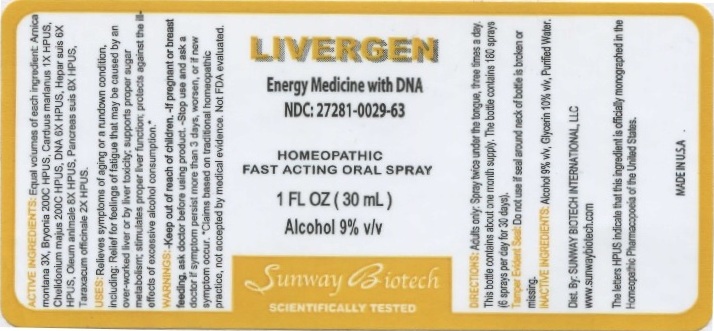
Label: LIVERGEN- arnica montana, bryonia, carduus marianus, chelidonium majus, dna, hepar suis, oleum animale, pancreas suis, taraxacum officinale. liquid
- NDC Code(s): 27281-029-63
- Packager: Sunway Biotech LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
ACTIVE INGREDIENTS: Equal volumes of each ingredient: Arnica montana 3X, Bryonia 200C HPUS, Carduus marianus 1X HPUS, Chelidonium majus 200C HPUS, DNA 6X HPUS, Hepar suis 6X HPUS, Oleum animale 8X HPUS, Pancreas suis 8X HPUS, Taraxacum officinale 2X HPUS.
The letters HPUS indicate that this ingredient is officially monographed in the Homeopathic Pharmacopeia of the United States.
-
INDICATIONS & USAGE
USES: Relieves symptoms of aging or a rundown condition, including: Relief for feelings of fatigue that may be caused by an over-worked liver or by liver toxixity; supports proper sugar metabolism; stimulates proper liver function; protects against the ill-effects of excessive alcohol consumption.*
*Claims based on traditional homeopathic practice, not accepted by medical evidence. Not FDA evaluated.
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIVERGEN
arnica montana, bryonia, carduus marianus, chelidonium majus, dna, hepar suis, oleum animale, pancreas suis, taraxacum officinale. liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:27281-029 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 3 [hp_X] in 30 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 200 [hp_C] in 30 mL MILK THISTLE (UNII: U946SH95EE) (MILK THISTLE - UNII:U946SH95EE) MILK THISTLE 1 [hp_X] in 30 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 200 [hp_C] in 30 mL HERRING SPERM DNA (UNII: 51FI676N6F) (HERRING SPERM DNA - UNII:51FI676N6F) HERRING SPERM DNA 6 [hp_X] in 30 mL PORK LIVER (UNII: 6EC706HI7F) (PORK LIVER - UNII:6EC706HI7F) PORK LIVER 6 [hp_X] in 30 mL CERVUS ELAPHUS HORN OIL (UNII: 7A7G0PQI12) (CERVUS ELAPHUS HORN OIL - UNII:7A7G0PQI12) CERVUS ELAPHUS HORN OIL 8 [hp_X] in 30 mL SUS SCROFA PANCREAS (UNII: 9Y3J3362RY) (SUS SCROFA PANCREAS - UNII:9Y3J3362RY) SUS SCROFA PANCREAS 8 [hp_X] in 30 mL TARAXACUM OFFICINALE (UNII: 39981FM375) (TARAXACUM OFFICINALE - UNII:39981FM375) TARAXACUM OFFICINALE 2 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:27281-029-63 30 mL in 1 PACKAGE; Type 0: Not a Combination Product 11/13/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/13/2013 Labeler - Sunway Biotech LLC (019560802)