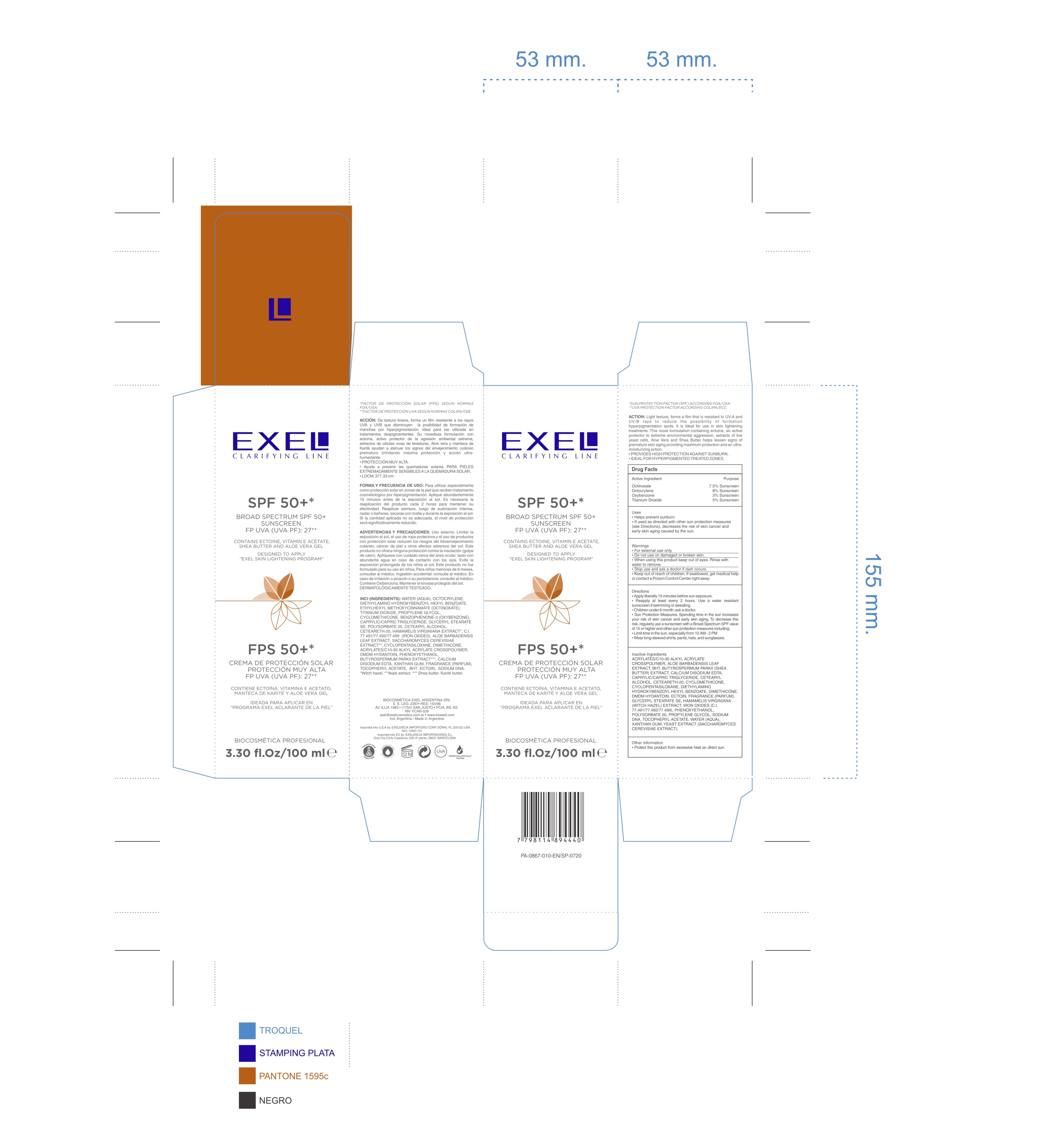
Label: EXEL CLARIFYING LINE SPF 50- octinoxate, octocrylene, oxybenzone, tatanium dioxide cream
- NDC Code(s): 53863-125-01, 53863-125-02
- Packager: Exelencia importers
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS AND PRECAUTIONS
-
DOSAGE & ADMINISTRATION
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Children under 6 month: ask a doctor
-Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, Especially from 10 AM - 2 PMWear long-sleeved shirts, pants, hats, and sunglassesUse a water resistant sunscreen if swimming or sweating
-
INACTIVE INGREDIENT
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Extract, BHT, Butyrospermum Parkii (shea butter) Extract, Calcium Disodium EDTA, Caprylic/Capric Triglyceride, Cetearyl Alcohol (and) Ceteareth-20, Cyclomethicone, Cyclopentasiloxane, Diethlyamino Hydroxybenzoyl Hexyl Benzoate, Dimethicone, DMDM Hydantoin, Ectoin, Fragrance (Parfum), Glyceryl Stearate SE, Hamamelis Virginiana (witch hazel) Extract, Iron Oxides (C.I. 77497/77492/77499), Phenoxyethanol, Polysorbate 20, Propylene Glycol, Sodium DNA, Tocopheryl Acetate, Water (Aqua), Xanthan Gum, Yeast Extract (saccharomyces cerevisiae extract)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EXEL CLARIFYING LINE SPF 50
octinoxate, octocrylene, oxybenzone, tatanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53863-125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 8 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 3 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SHEANUT OIL (UNII: O88E196QRF) EDETATE CALCIUM DISODIUM ANHYDROUS (UNII: 8U5D034955) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CYCLOMETHICONE (UNII: NMQ347994Z) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) DIMETHICONE (UNII: 92RU3N3Y1O) DMDM HYDANTOIN (UNII: BYR0546TOW) ECTOINE (UNII: 7GXZ3858RY) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53863-125-02 1 in 1 PACKAGE 05/14/2015 1 NDC:53863-125-01 100 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 05/14/2015 Labeler - Exelencia importers (147644335) Establishment Name Address ID/FEI Business Operations Biocosmetica Exel Argentina SRL 971813332 manufacture(53863-125)