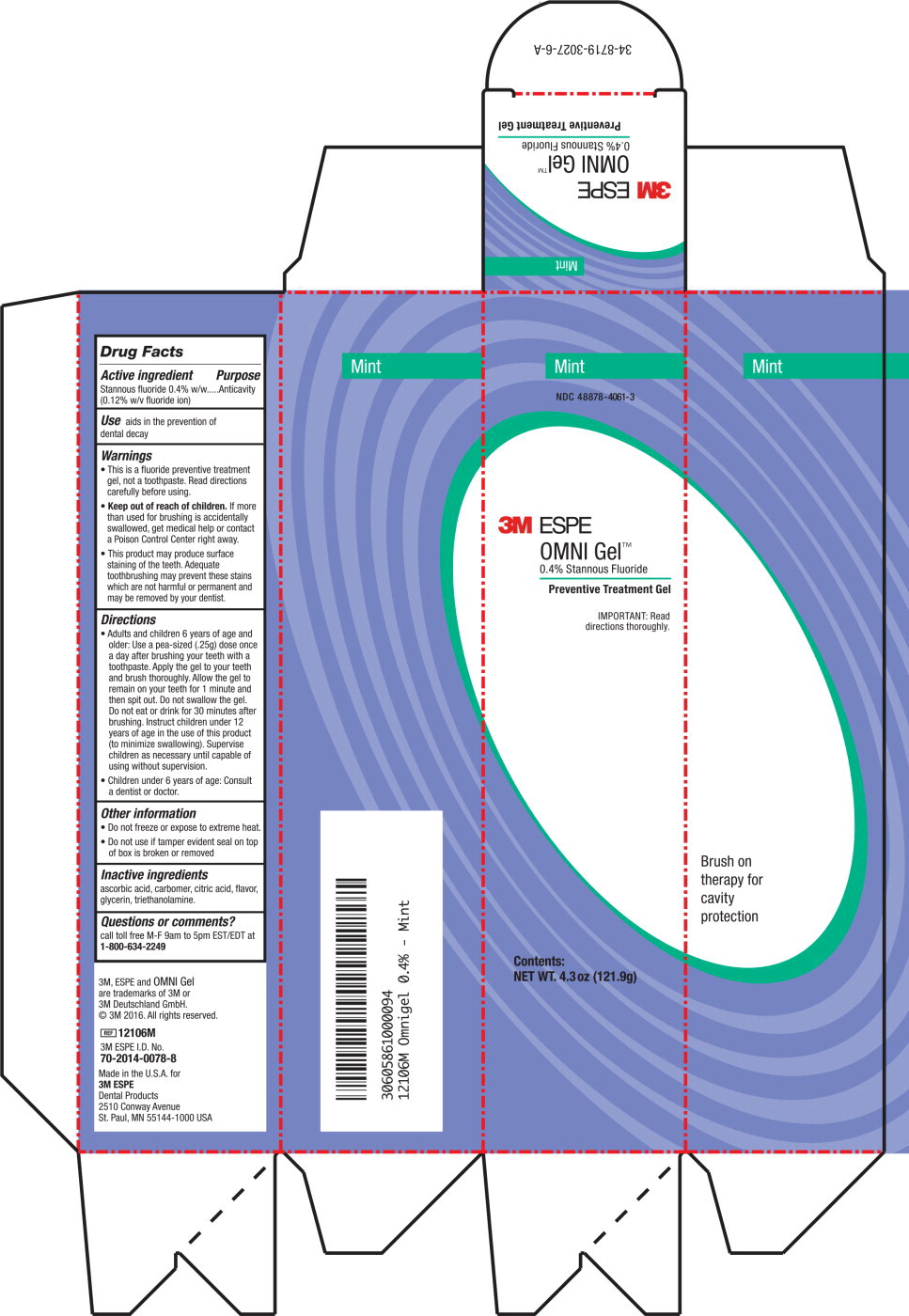
Label: OMNI- stannous fluoride gel
- NDC Code(s): 48878-4061-3
- Packager: Solventum US OpCo LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
- This is a fluoride preventive treatment gel, not a toothpaste. Read directions carefully before using.
- Keep out of reach of children. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
- This product may produce surface staining of the teeth. Adequate tooth brushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
-
Directions
- Adults and children 6 years of age and older: Use a pea-sized (.25g) dose once a day after brushing your teeth with a toothpaste. Apply the gel to your teeth and brush thoroughly. Allow the gel to remain on your teeth for 1 minute and then spit out. Do not swallow the gel. Do not eat or drink for 30 minutes after brushing. Instruct children under 12 years of age in the use of this product (to minimize swallowing). Supervise children as necessary until capable of using without supervision.
- Children under 6 years of age: Consult a dentist or doctor.
- Other information
- Inactive ingredients
- Questions or comments?
- Principle Display Panel
-
INGREDIENTS AND APPEARANCE
OMNI
stannous fluoride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:48878-4061 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Stannous Fluoride (UNII: 3FTR44B32Q) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 0.969 mg in 1 g Inactive Ingredients Ingredient Name Strength Ascorbic Acid (UNII: PQ6CK8PD0R) Carbomer Homopolymer Type B (Allyl Sucrose Crosslinked) (UNII: Z135WT9208) Anhydrous Citric Acid (UNII: XF417D3PSL) Glycerin (UNII: PDC6A3C0OX) Trolamine (UNII: 9O3K93S3TK) Product Characteristics Color Score Shape Size Flavor MINT (MINT) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:48878-4061-3 1 in 1 BOX 02/01/1998 1 121.9 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 02/01/1998 Labeler - Solventum US OpCo LLC (801390852)