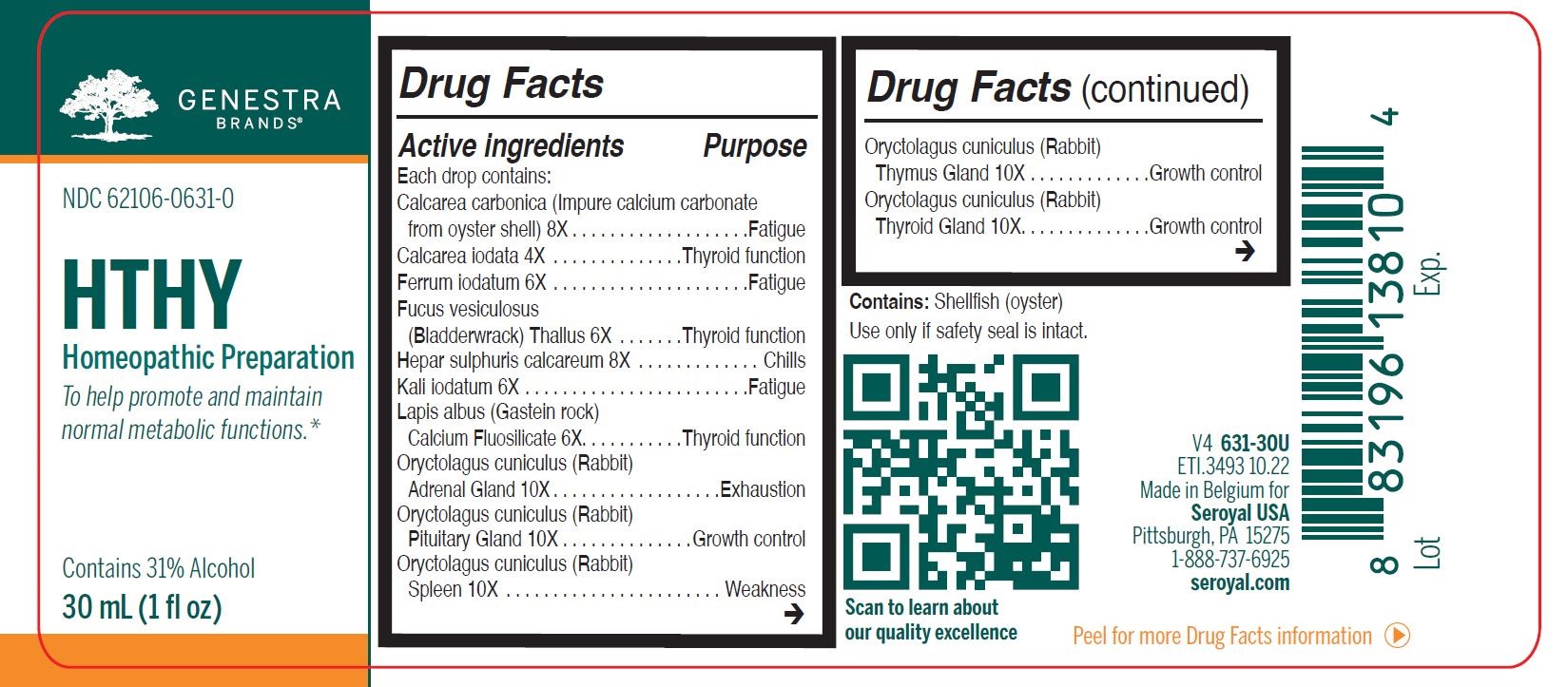
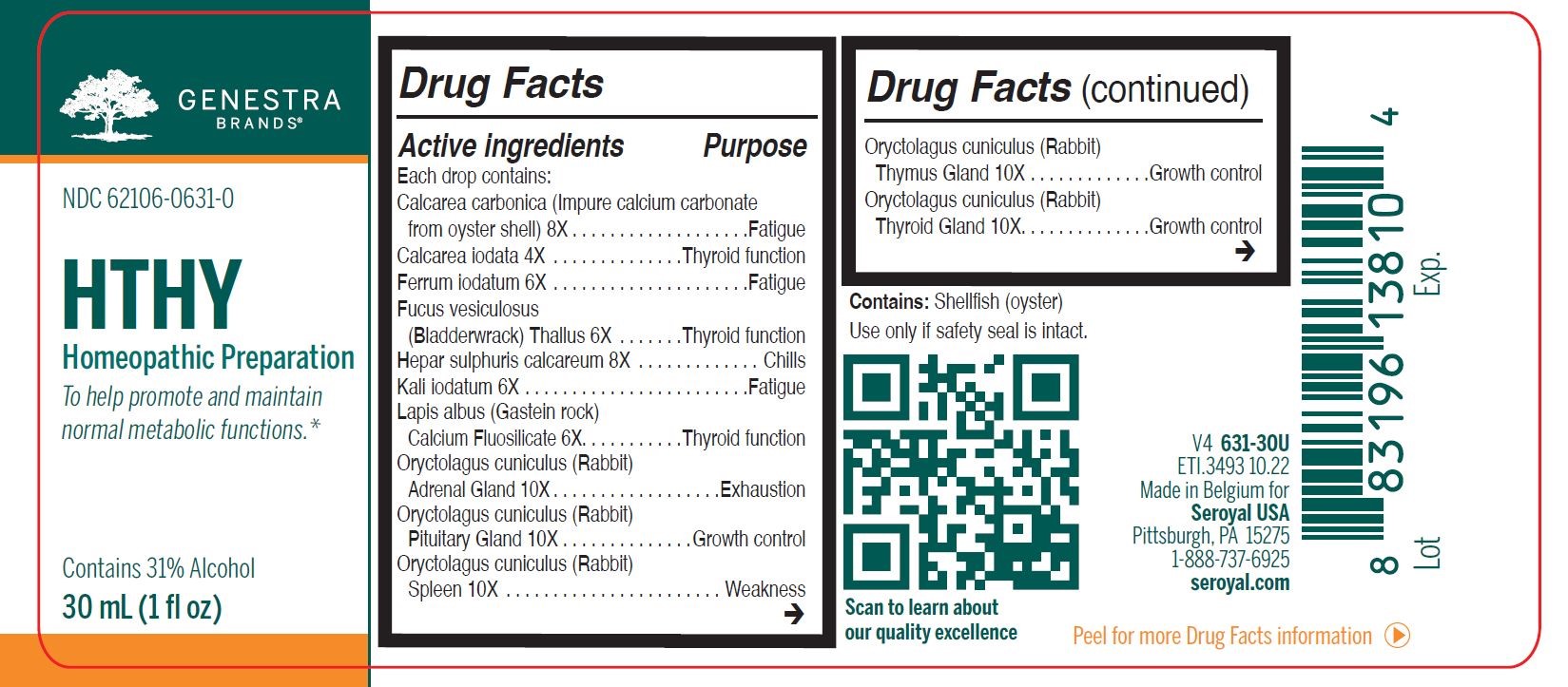
Label: HTHY (calcarea carbonica, calcarea iodata, ferrum iodatum, fucus vesiculosus, hepar sulphuris calcareum, kali iodatum, lapis albus calcium fluosilicate, oryctolagus cuniculus (rabbit) adrenal gland, oryctolagus cuniculus (rabbit) pituitary gland, oryctolagus cuniculus (rabbit) spleen, oryctolagus cuniculus (rabbit) thymus gland, oryctolagus cuniculus- rabbit thyroid gland, spongia tosta liquid
- NDC Code(s): 62106-0631-0
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients
Each drop contains:
Calcarea carbonica 8X
Calcarea iodata 4X
Ferrum iodatum 6X
Fucus vesiculosus (Bladderwrack) Thallus 6X
Hepar sulphuris calcareum 8X
Kali iodatum 6X
Lapis albus (Gastein rock) Calcium Fluosilicate 6X
Oryctolagus cuniculus (Rabbit) Adrenal Gland 10X
Oryctolagus cuniculus (Rabbit) Pituitary Gland 10XOryctolagus cuniculus (Rabbit) Spleen 10X
Oryctolagus cuniculus (Rabbit) Thymus Gland 10XOryctolagus cuniculus (Rabbit) Thyroid Gland 10X
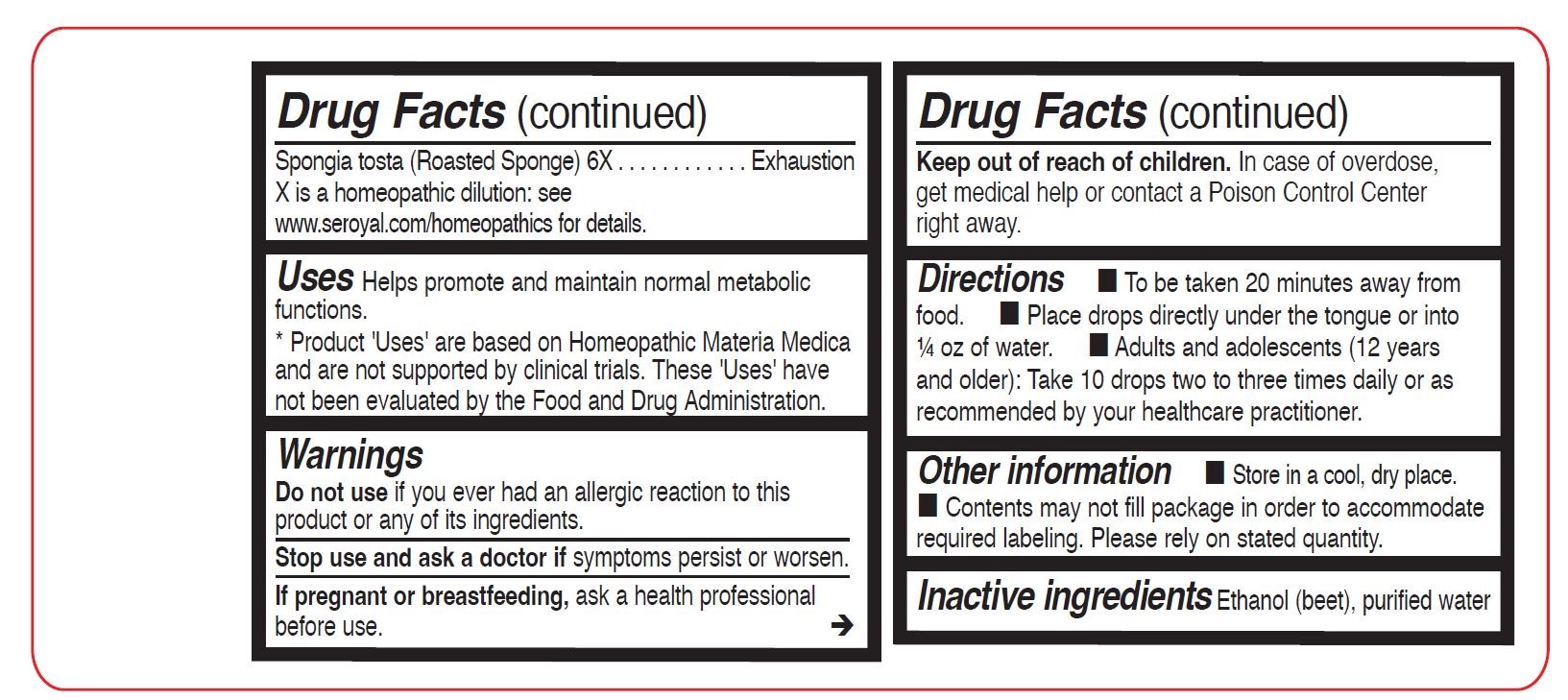
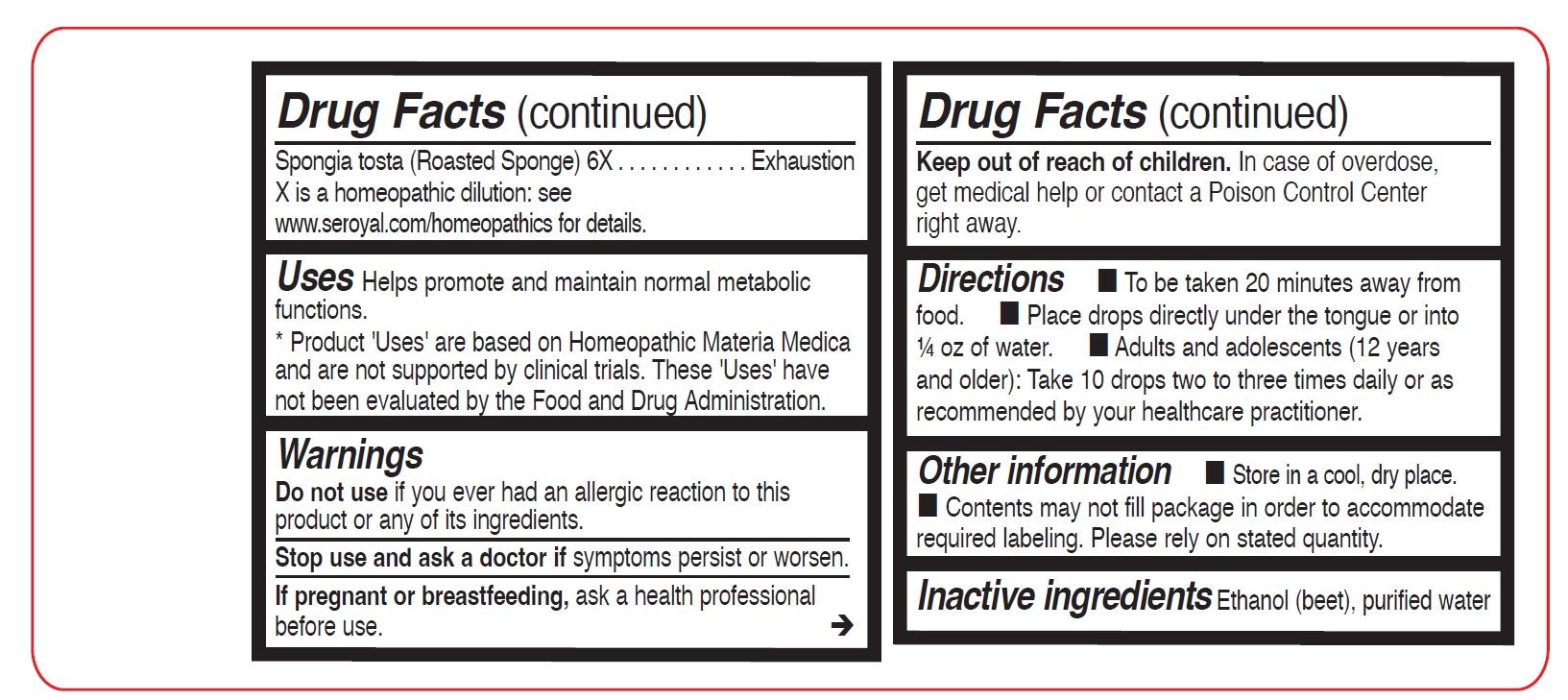
Spongia tosta (Roasted Sponge) 6X - PURPOSE
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Directions
To be taken 20 minutes away from food.
Place drops directly under the tongue or into ¼ oz of water.
Adults and adolescents (12 years and older): Take 10 drops two to three times daily or as recommended
by your healthcare practitioner.Children (under 12 years): Take under the direction of your healthcare practitioner.
-
INDICATIONS & USAGE
Uses
Helps promote and maintain normal metabolic functions.
Directions
To be taken 20 minutes away from food.
Place drops directly under the tongue or into ¼ oz of water.
Adults and adolescents (12 years and older): Take 10 drops two to three times daily or as recommended
by your healthcare practitioner.Children (under 12 years): Take under the direction of your healthcare practitioner.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HTHY
calcarea carbonica, calcarea iodata, ferrum iodatum, fucus vesiculosus, hepar sulphuris calcareum, kali iodatum, lapis albus calcium fluosilicate, oryctolagus cuniculus (rabbit) adrenal gland, oryctolagus cuniculus (rabbit) pituitary gland, oryctolagus cuniculus (rabbit) spleen, oryctolagus cuniculus (rabbit) thymus gland, oryctolagus cuniculus (rabbit) thyroid gland, spongia tosta liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-0631 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 8 [hp_X] in 30 mL CALCIUM IODIDE (UNII: 8EKI9QEE2H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM IODIDE 4 [hp_X] in 30 mL FERROUS IODIDE (UNII: F5452U54PN) (FERROUS IODIDE - UNII:F5452U54PN) FERROUS IODIDE 6 [hp_X] in 30 mL FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 6 [hp_X] in 30 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 8 [hp_X] in 30 mL CALCIUM HEXAFLUOROSILICATE (UNII: 2NVP93XVQ3) (CALCIUM HEXAFLUOROSILICATE - UNII:2NVP93XVQ3) CALCIUM HEXAFLUOROSILICATE 6 [hp_X] in 30 mL ORYCTOLAGUS CUNICULUS ADRENAL GLAND (UNII: QGY289M89Q) (ORYCTOLAGUS CUNICULUS ADRENAL GLAND - UNII:QGY289M89Q) ORYCTOLAGUS CUNICULUS ADRENAL GLAND 10 [hp_X] in 30 mL ORYCTOLAGUS CUNICULUS PITUITARY GLAND (UNII: T9F9ZW799A) (ORYCTOLAGUS CUNICULUS PITUITARY GLAND - UNII:T9F9ZW799A) ORYCTOLAGUS CUNICULUS PITUITARY GLAND 10 [hp_X] in 30 mL ORYCTOLAGUS CUNICULUS SPLEEN (UNII: 01WNS05SFO) (ORYCTOLAGUS CUNICULUS SPLEEN - UNII:01WNS05SFO) ORYCTOLAGUS CUNICULUS SPLEEN 10 [hp_X] in 30 mL ORYCTOLAGUS CUNICULUS THYMUS (UNII: 7ZO132YX2X) (ORYCTOLAGUS CUNICULUS THYMUS - UNII:7ZO132YX2X) ORYCTOLAGUS CUNICULUS THYMUS 10 [hp_X] in 30 mL SPONGIA OFFICINALIS SKELETON, ROASTED (UNII: 1PIP394IID) (SPONGIA OFFICINALIS SKELETON, ROASTED - UNII:1PIP394IID) SPONGIA OFFICINALIS SKELETON, ROASTED 6 [hp_X] in 30 mL POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) POTASSIUM IODIDE 6 [hp_X] in 30 mL ORYCTOLAGUS CUNICULUS THYROID (UNII: 97K9E28344) (ORYCTOLAGUS CUNICULUS THYROID - UNII:97K9E28344) ORYCTOLAGUS CUNICULUS THYROID 10 [hp_X] in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-0631-0 1 in 1 CARTON 11/27/2015 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/27/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN'UP 401010287 manufacture(62106-0631)