A1181-18 CONTINUOUS EPIDURAL 18G HUSTEAD- regional anesthesia kit
Smiths Medical ASD, Inc.
----------
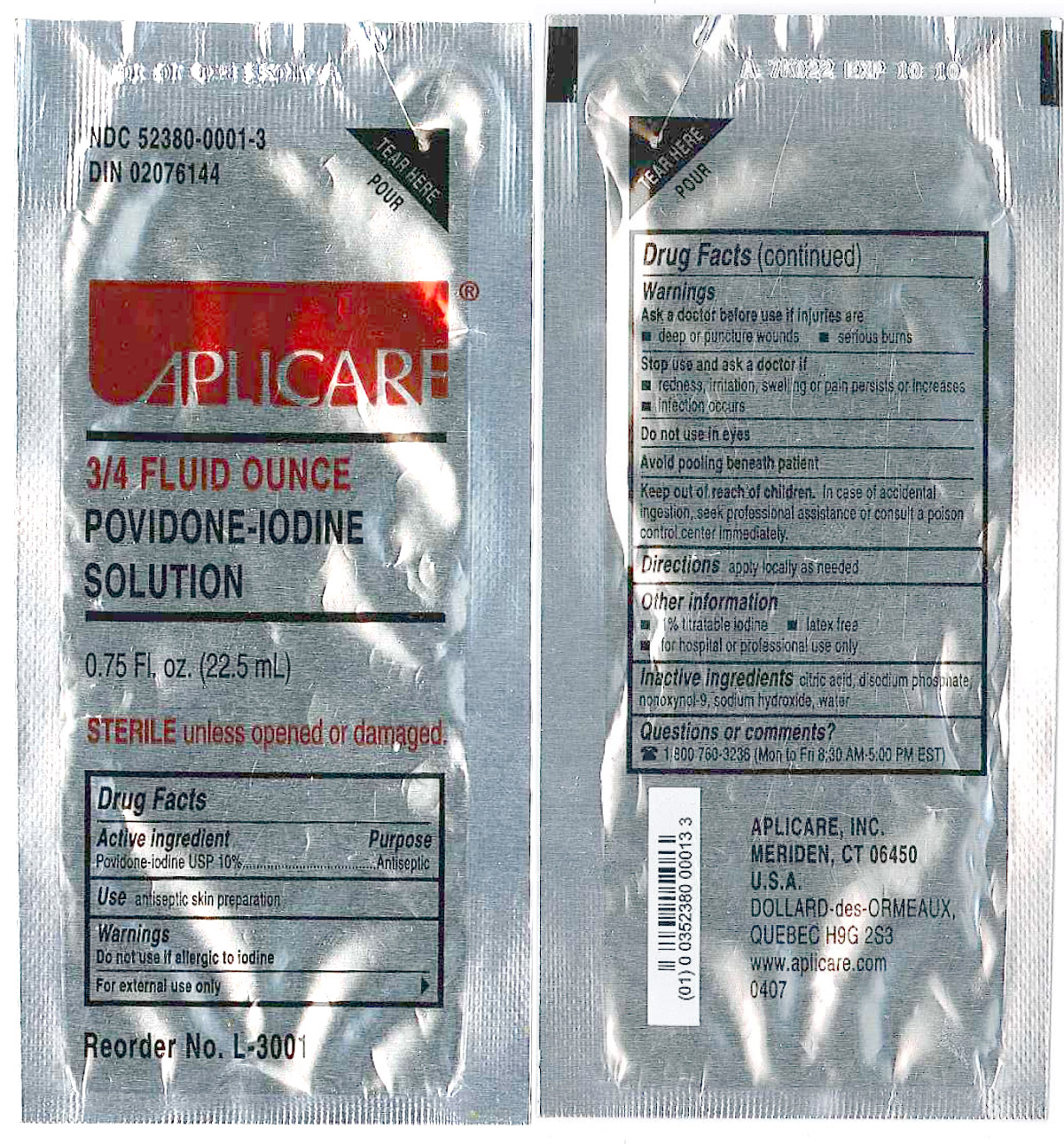
APLICARE POVIDONE-IODINE SOLUTION
(povidone-iodine solution)
solution
[Aplicare, Inc.]
3/4 Ounce Povidone Iodine Packet
Povidone-iodine 10%
Antiseptic
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children.In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.
| A1181-18 CONTINUOUS EPIDURAL 18G HUSTEAD
regional anesthesia kit kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Smiths Medical ASD, Inc. (137835299) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Smiths Medical ASD, Inc. | 137835299 | relabel, manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aplicare, Inc. | 081054904 | manufacture | |
Revised: 2/2020
Document Id: f1715948-8600-409d-8d9d-df6cef5385a6
Set id: 603471b2-df49-4179-ae35-42a2cb9b810c
Version: 2
Effective Time: 20200219
Smiths Medical ASD, Inc.