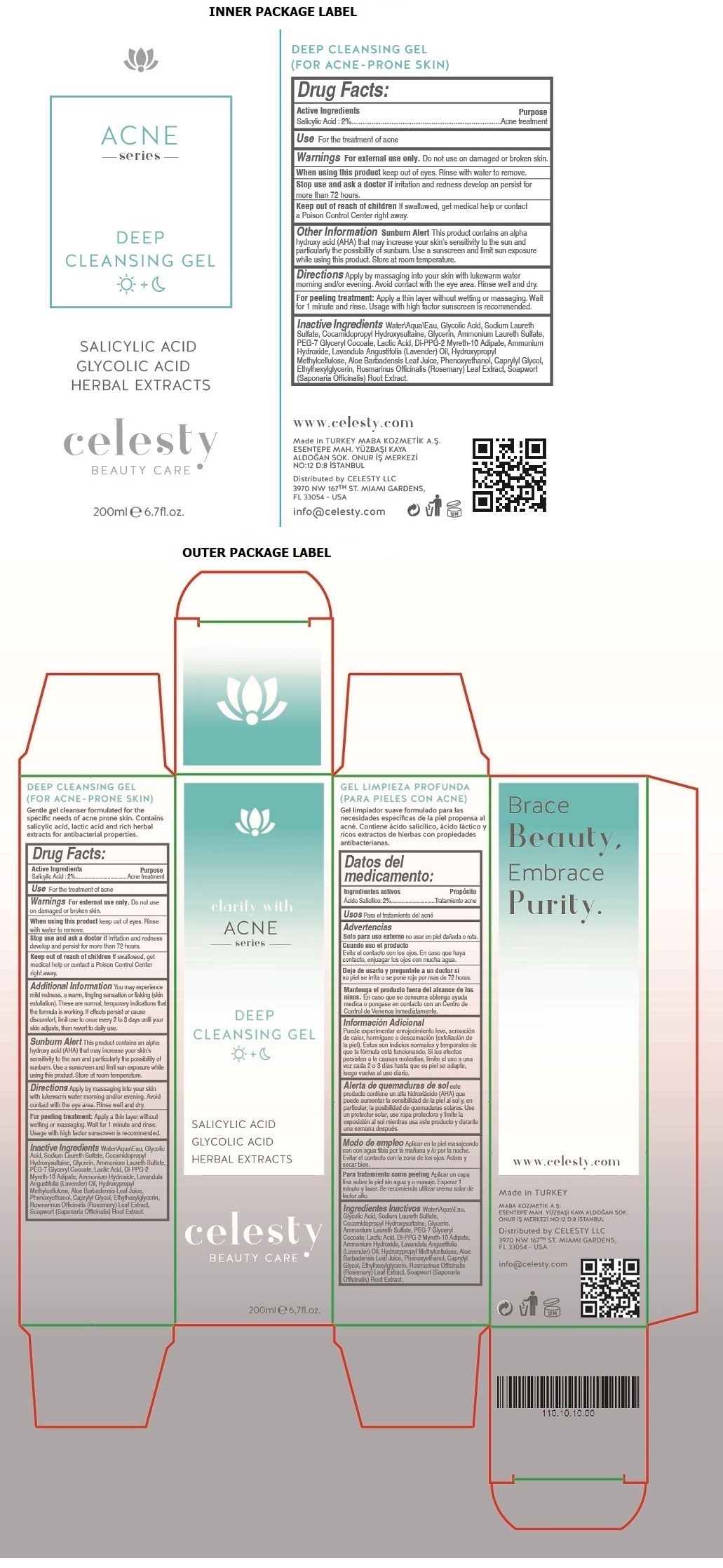
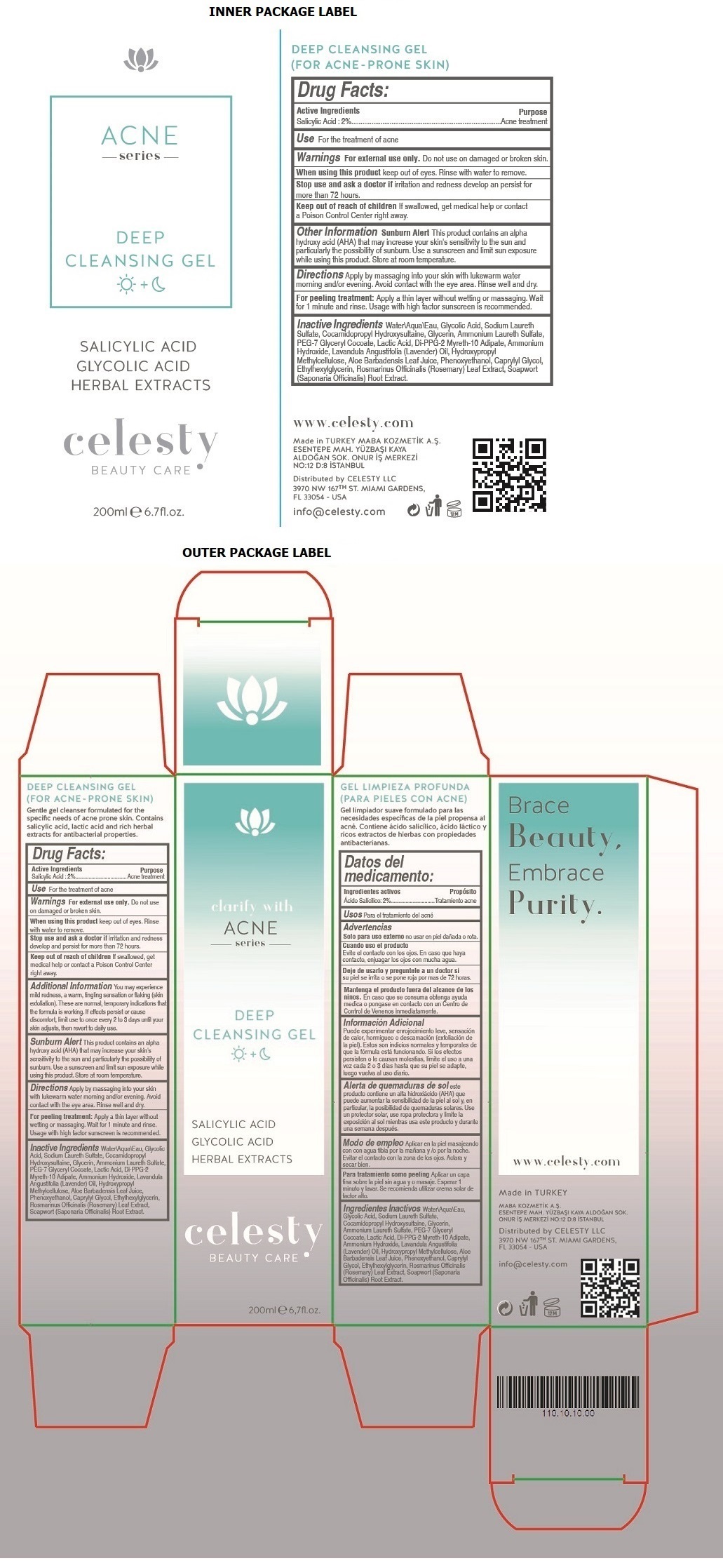
Label: CELESTY ACNE SERIES DEEP CLEANSING GEL- salicylic acid gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 81120-102-01 - Packager: MABA KOZMETIK LIMITED SIRKETI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 7, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts:
- Active Ingredients
- Purpose
- Use
- Warnings
-
Additional Information
You may experience mild redness, a warm, tingling sensation or flaking (skin exfoliation). These are normal, temporary indications that the formula is working. If effects persist or cause discomfort, limit use to once every 2 to 3 days until your skin adjusts, then revert to daily use.
Sunburn Alert This product contains an alpha hydroxy acid (AHA) that may increase your skin's sensitivity to the sun and particularly the possibility of sunburn. Use a sunscreen and limit sun exposure while using this product. Store at room temperature.
- Directions
-
Inactive Ingredients
Water\Aqua\Eau, Glycolic Acid, Sodium Laureth Sulfate, Cocamidopropyl Hydroxysultaine, Glycerin, Ammonium Laureth Sulfate, PEG-7 Glyceryl Cocoate, Lactic Acid, Di-PPG-2 Myreth-10 Adipate, Ammonium Hydroxide, Lavandula Angustifolia (Lavender) Oil, Hydroxypropyl Methylcellulose, Aloe Barbadensis Leaf Juice, Phenoxyethanol, Caprylyl Glycol, Ethylhexylglycerin, Rosmarinus Officinalis (Rosemary) Leaf Extract, Soapwort (Saponaria Officinalis) Root Extract
-
SPL UNCLASSIFIED SECTION
clarify with ACNE series
SALICYLIC ACID
GLYCOLIC ACID
HERBAL EXTRACTS
BEAUTY CARE
(FOR ACNE - PRONE SKIN)
Gentle gel cleanser formulated for the specific needs of acne prone skin. Contains salicylic acid, lactic acid and rich herbal extracts for antibacterial properties.
Brace Beauty, Embrace Purity.
www.celesty.com
Made in TURKEY
MABA KOZMETIK A.S.
ESENTEPE MAH. YUZBASI KAYA ALDOGAN SOK.
ONUR IS MERKEZI NO:12 D:8 ISTANBUL
Distributed by CELESTY LLC
3970 NW 167TH ST. MIAMI GARDENS,
FL 33054 - USA
info@celesty.com
- Packaging
-
INGREDIENTS AND APPEARANCE
CELESTY ACNE SERIES DEEP CLEANSING GEL
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81120-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCOLIC ACID (UNII: 0WT12SX38S) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) GLYCERIN (UNII: PDC6A3C0OX) AMMONIUM LAURETH-2 SULFATE (UNII: 698O4Z48G6) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) DI-PPG-2 MYRETH-10 ADIPATE (UNII: 4IN301M0KJ) AMMONIA (UNII: 5138Q19F1X) LAVENDER OIL (UNII: ZBP1YXW0H8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ALOE VERA LEAF (UNII: ZY81Z83H0X) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ROSEMARY (UNII: IJ67X351P9) SAPONARIA OFFICINALIS ROOT (UNII: RI2K1BMA8B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81120-102-01 1 in 1 BOX 12/07/2020 1 200 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 12/07/2020 Labeler - MABA KOZMETIK LIMITED SIRKETI (503001418) Establishment Name Address ID/FEI Business Operations MABA KOZMETIK LIMITED SIRKETI 503001418 manufacture(81120-102)