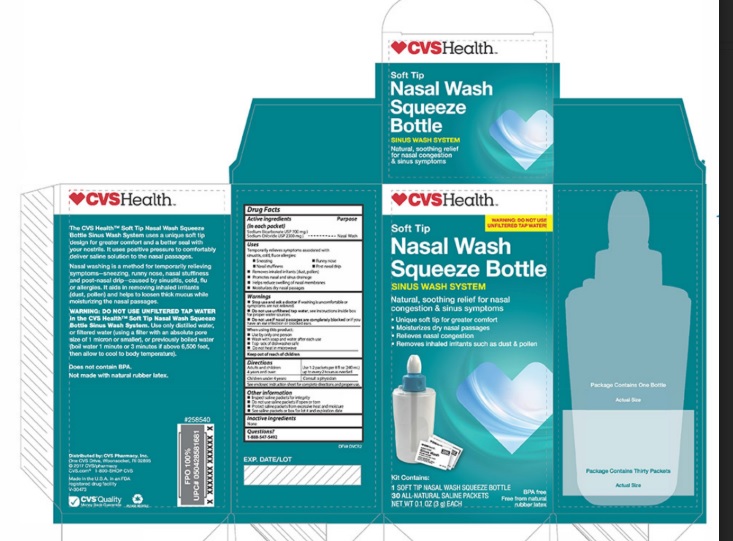
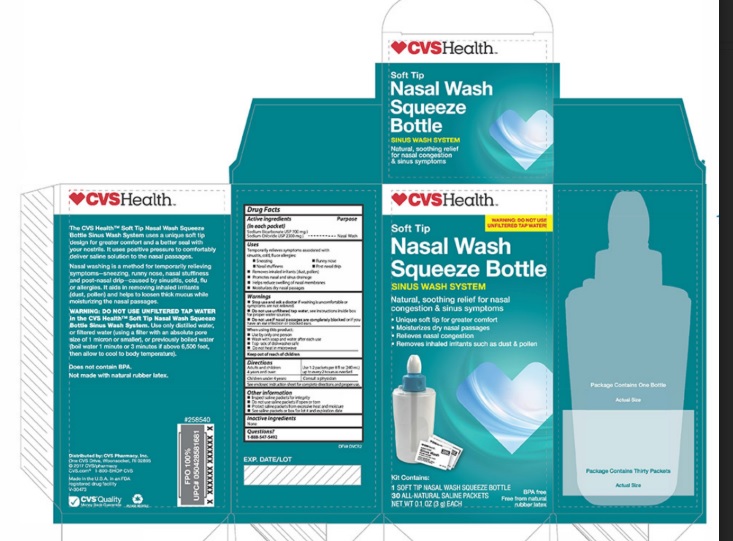
Label: CVS- sodium chloride, sodium bicarbonate kit
- NDC Code(s): 69842-324-01, 69842-453-10
- Packager: CVS Pharmacy, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
• Stop use and ask a doctor if washing is uncomfortable or
symptoms are not relieved
• Do not use unfiltered tap water, see instructions inside box
for proper water sources.
• Do not use if nasal passages are completely blocked or if you
have an ear infection or blocked ears.
When using this product:
- Use by only one person
- Wash with soap and water after each use
- Top rack of dishwasher safe
- Do not heat in microwave
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PATIENT PACKAGE INSERT
- PATIENT PACKAGE INSERT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS
sodium chloride, sodium bicarbonate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-453 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-453-10 1 in 1 KIT 04/24/2017 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 3000 mg Part 1 of 1 CVS
sodium bicarbonate, sodium chloride powderProduct Information Item Code (Source) NDC:69842-324 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM BICARBONATE (UNII: 8MDF5V39QO) (BICARBONATE ION - UNII:HN1ZRA3Q20) SODIUM BICARBONATE 22.8 mg in 100 mg SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 77.8 mg in 100 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 77.8 mg in 100 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 in 1 KIT 1 NDC:69842-324-01 3000 mg in 1 PACKET; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/24/2017 Labeler - CVS Pharmacy, Inc. (062312574)