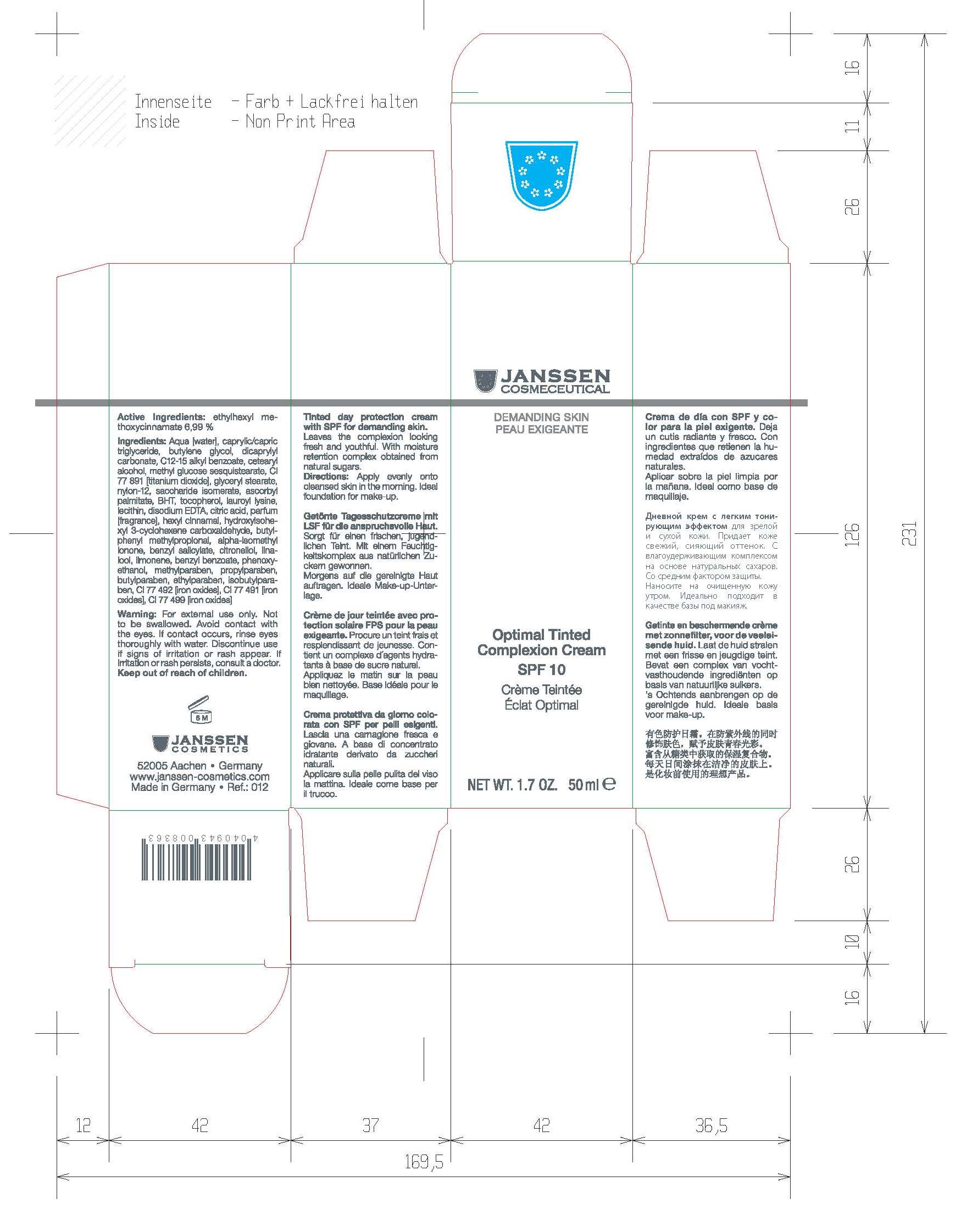
Label: OPTIMAL TINTED COMPLEXION- ethylhexl methoxycinnamate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 24653-011-01, 24653-011-02 - Packager: Janssen Cosmetics GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 20, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active Ingredients: ethylhexyl methoxycinnamate 7.0% Purpose: Sunscreen
Inactive Ingredients
Inactive Ingredients: Aqua [water], caprylic/capric triglyceride, butylene glycol, dicaprylyl carbonate, C12-15 alkyl benzoate, cetearyl
alcohol, methyl glucose sesquistearate, CI 77891 [titanium dioxide], glyceryl stearate, nylon-12, saccharide isomerate, ascorbyl
palmitate, BHT, tocopherol, lauroyl lysine, lecithin, disodium EDTA, citric acid, parfum [fragrance], hexyl cinnamal, hydroxyisohexyl 3-cyclohexene carboxaldehyde, butylphenyl methylpropional, alpha-isomethylionone, benzyl salicylate, citronellol, linalool, limonene, benzyl benzoate, phenoxyethanol, methylparaben, propylparaben,butylparaben, ethylparaben, isobutylparaben, CI 77 492 [iron oxides], CI 77 491 [iron oxides], CI 77 499 [iron oxides]
Warning Section
Avoid contact with eyes. Keep out of reach of children. For External use only. Does not replace the use of sun products in care of sun exposure
- Principal Display
- Principal Display Product Tube
-
INGREDIENTS AND APPEARANCE
OPTIMAL TINTED COMPLEXION
ethylhexl methoxycinnamate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24653-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.5 g in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) C12-15 ALKYL BENZOATE (UNII: A9EJ3J61HQ) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLUCAMETACIN (UNII: N1EXE5EHAN) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) NYLON-12 (UNII: 446U8J075B) SACCHARIDE ISOMERATE (UNII: W8K377W98I) LAUROYL LYSINE (UNII: 113171Q70B) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TOCOPHEROL (UNII: R0ZB2556P8) EDETATE DISODIUM (UNII: 7FLD91C86K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) BENZYL SALICYLATE (UNII: WAO5MNK9TU) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) LINALOOL, (+/-)- (UNII: D81QY6I88E) BENZYL BENZOATE (UNII: N863NB338G) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24653-011-02 1 in 1 BOX 1 NDC:24653-011-01 50 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/20/2012 Labeler - Janssen Cosmetics GmbH (499187946) Registrant - Janssen Cosmetics GmbH (499187946) Establishment Name Address ID/FEI Business Operations Janssen Cosmetics GmbH 499187946 manufacture(24653-011)