Label: SALICYLIC ACID CLEANSER- exfoliating and clarifying face wash gel
- NDC Code(s): 73318-7001-2, 73318-7001-4
- Packager: Skin PS Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
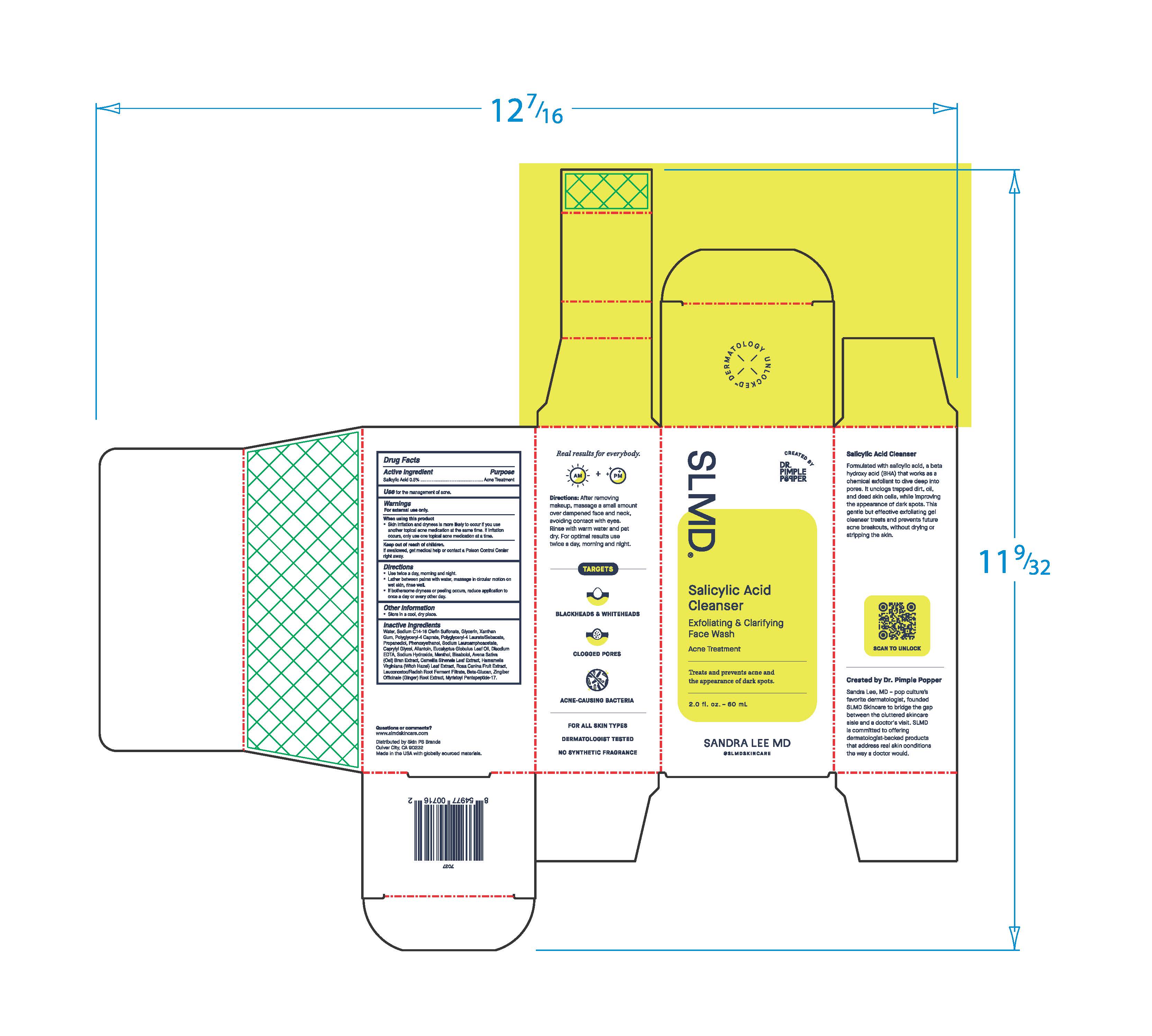
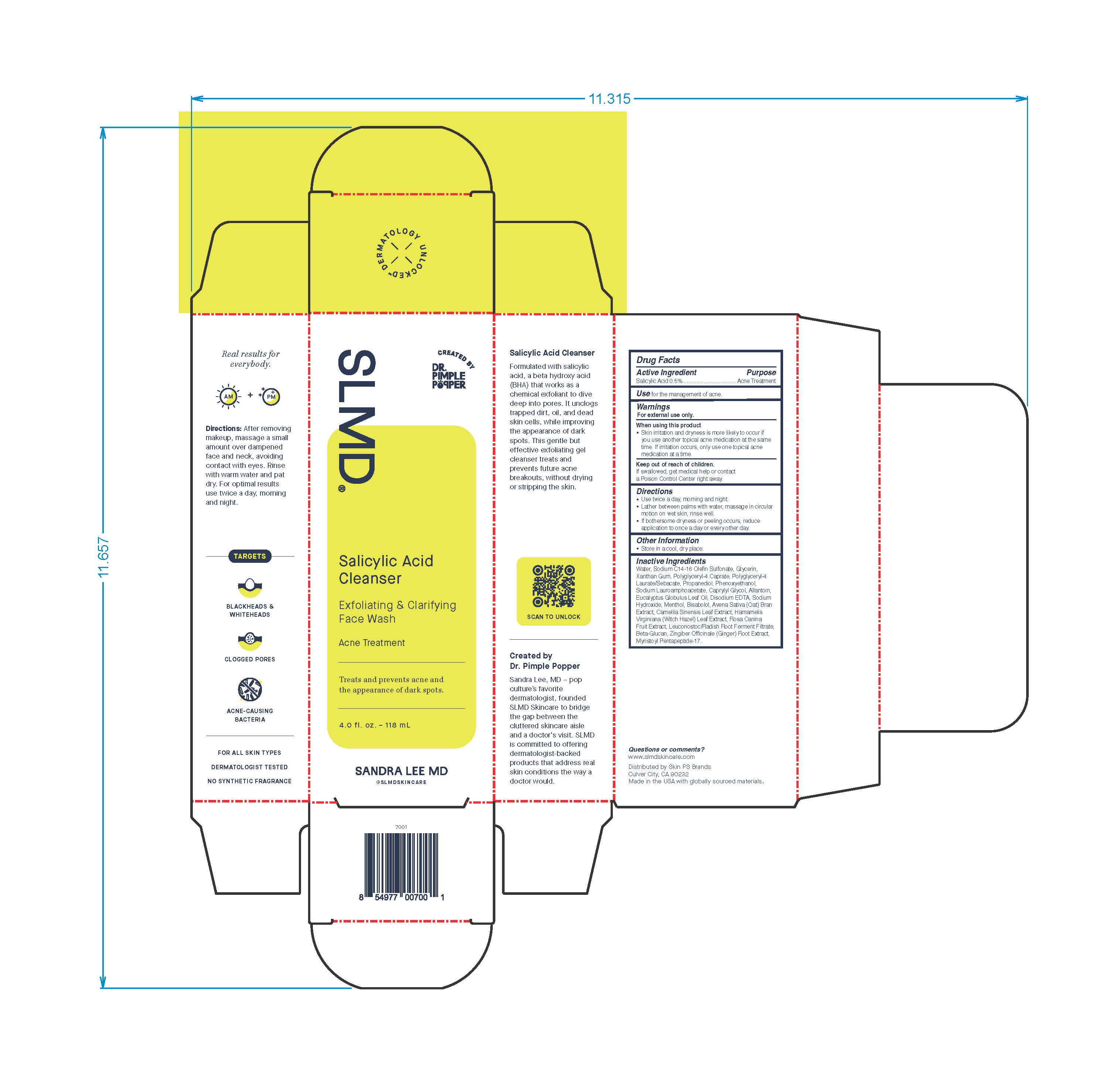
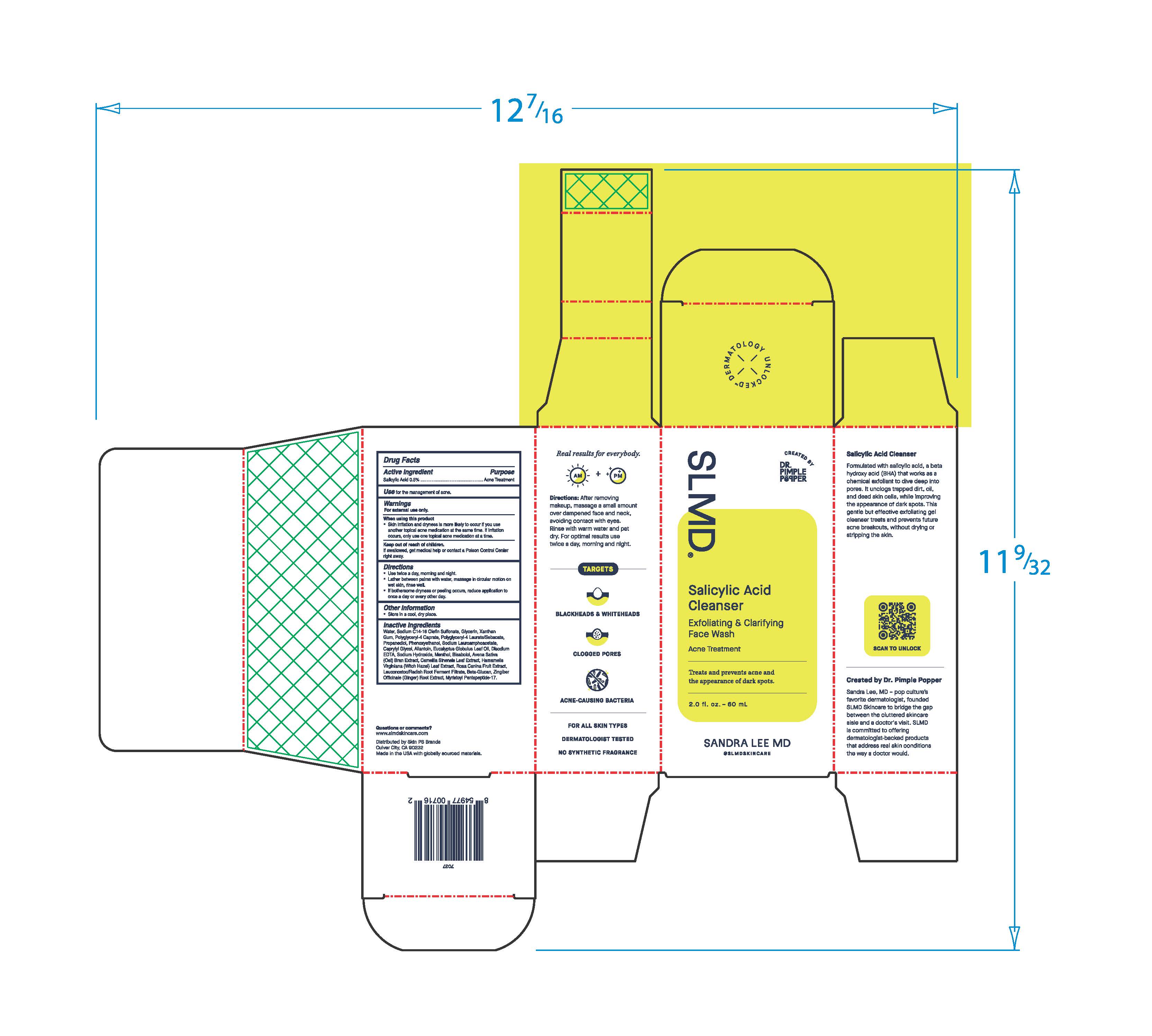
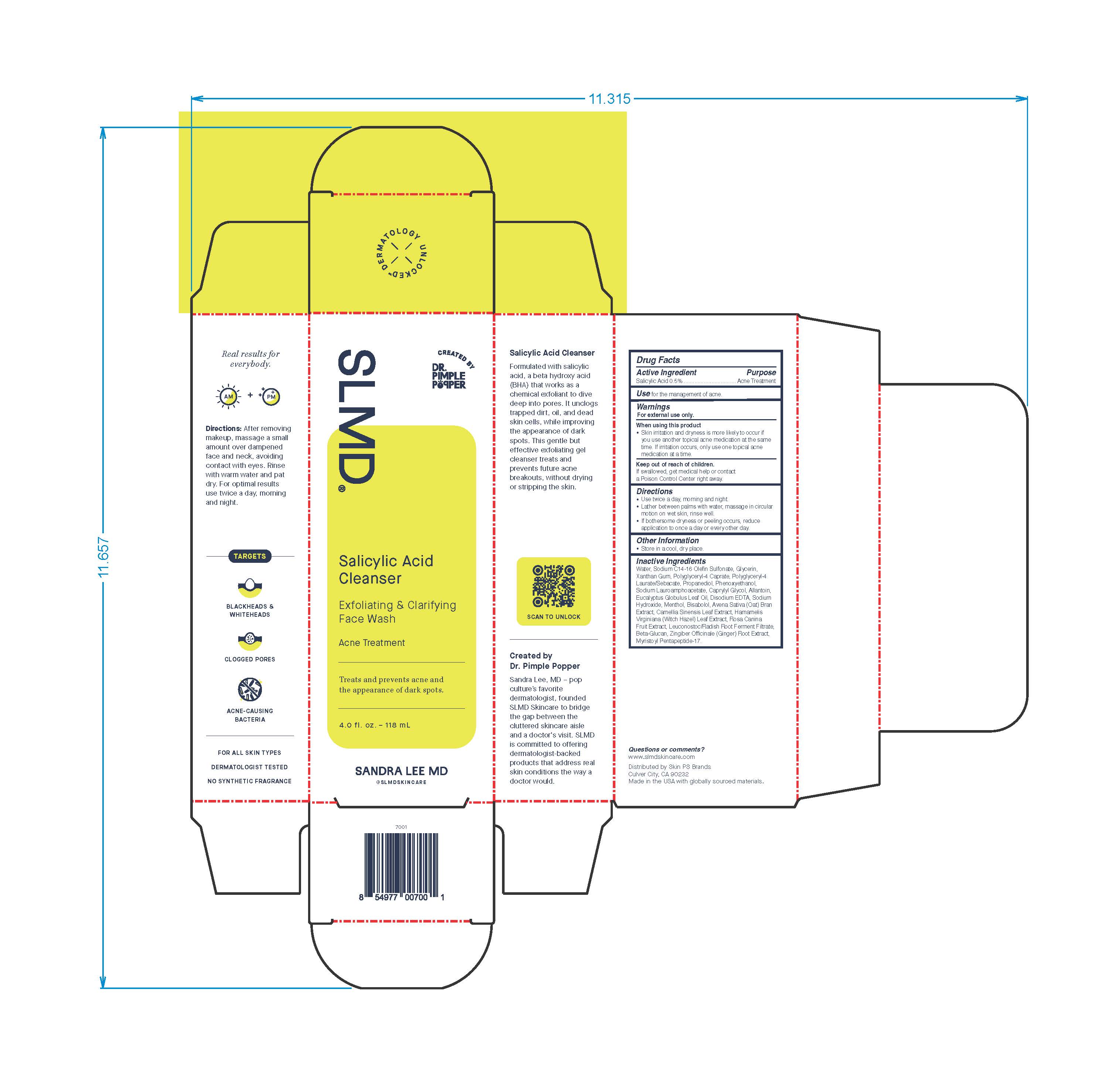
- Active Ingredient
- Purpose
- Use
- Warnings
- When using this product
- Keep out of reach of children.
- Directions
- Other Information
-
Inactive Ingredients
Water, Sodium C14-16 Olefin Sulfonate, Glycerin, Xanthan Gum, Polyglyceryl-4 Caprate, Polyglyceryl-4 Laurate/Sebacate, Propanediol, Phenoxyethanol, Sodium Lauroamphoacetate, Caprylyl Glycol, Allantoin, Eucalyptus Globulus Leaf Oil, Disodium EDTA, Sodium Hydroxide, Menthol, Bisabolol, Avena Sative (Oat) Bran Extract, Camellia Sinensis Leaf Extract, Hamamelis Virginiana (Witch Hazel) Leaf Extract, Rosa Canina Fruit Extract, Leuconostoc/Radish Root Ferment Filtrate, Beta-Glucan, Zingiber Officinale (Ginger) Root Extract, Myristoyl Pentapeptide-17.
- Questions or comments?
-
Real results for everbody.
Directions: After removing makeup, massage a small amount over dampened face and neck avoiding contact with eyes. Rinse with warm water and pat dry. For optimal results use twice a day, morning and night.
TARGETS
BLACKHEADS & WHITEHEADS
CLOGGED PORES
ACNE-CAUSING BACTERIA
FOR ALL SKIN TYPES
DERMATOLOGIST TESTED
NON SYNTHETIC FRAGRANCE
-
Salicylic Acid Cleanser
Formulated with salicylic acid, a beta hydroxy acid (BHA) that works as a chemical exfoliant to dive deep into pores. It unclogs trapped dirt, oil, and dead skin cells, while improving the appearance of dark spots. This gentle but effective exfoliating gel cleanser treats and prevents future acne breakouts, without drying or stripping the skin.
Created by Dr. Pimple Popper
Sandra Lee, MD - pop culture's favorited dermatologist, founded SLMD Skincare to bridge the gap between the cluttered skincare aisle and a doctor's visit. SLMD is committed to offering dermatologist-backed products that address real skin conditions the way a doctor would.
- SLMD
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID CLEANSER
exfoliating and clarifying face wash gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73318-7001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 5 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM LAUROAMPHOACETATE (UNII: SLK428451L) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) AVENA SATIVA WHOLE (UNII: 5P8D0Z74RG) HAMAMELIS VIRGINIANA LEAF (UNII: T07U1161SV) ZINGIBER OFFICINALE WHOLE (UNII: IN6Q3S3414) MENTHOL (UNII: L7T10EIP3A) CAMELLIA SINENSIS WHOLE (UNII: C5M4585ZBZ) POLYGLYCERYL-4 CAPRATE (UNII: 3N873UN885) ALLANTOIN (UNII: 344S277G0Z) WATER (UNII: 059QF0KO0R) POLYGLYCERYL-4 LAURATE (UNII: 82V7NG3DYT) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM CARBOXYMETHYL .BETA.-GLUCAN (DS 0.65-0.85) (UNII: 2YGO1190AP) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) PROPANEDIOL (UNII: 5965N8W85T) EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) ROSA CANINA FRUIT (UNII: 3TNW8D08V3) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BISABOLOL OXIDE A (UNII: 16AE65F94Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73318-7001-2 1 in 1 CARTON 07/01/2023 1 60 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:73318-7001-4 1 in 1 CARTON 07/01/2023 2 118 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 07/01/2023 Labeler - Skin PS Brands (081085221) Registrant - Skin PS Brands (081085221) Establishment Name Address ID/FEI Business Operations Bentley Laboratories 068351753 manufacture(73318-7001)