Label: MINITRAN- nitroglycerin patch
- NDC Code(s): 0089-0171-02, 0089-0172-02, 0089-0173-02
- Packager: Kindeva Drug Delivery L.P.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
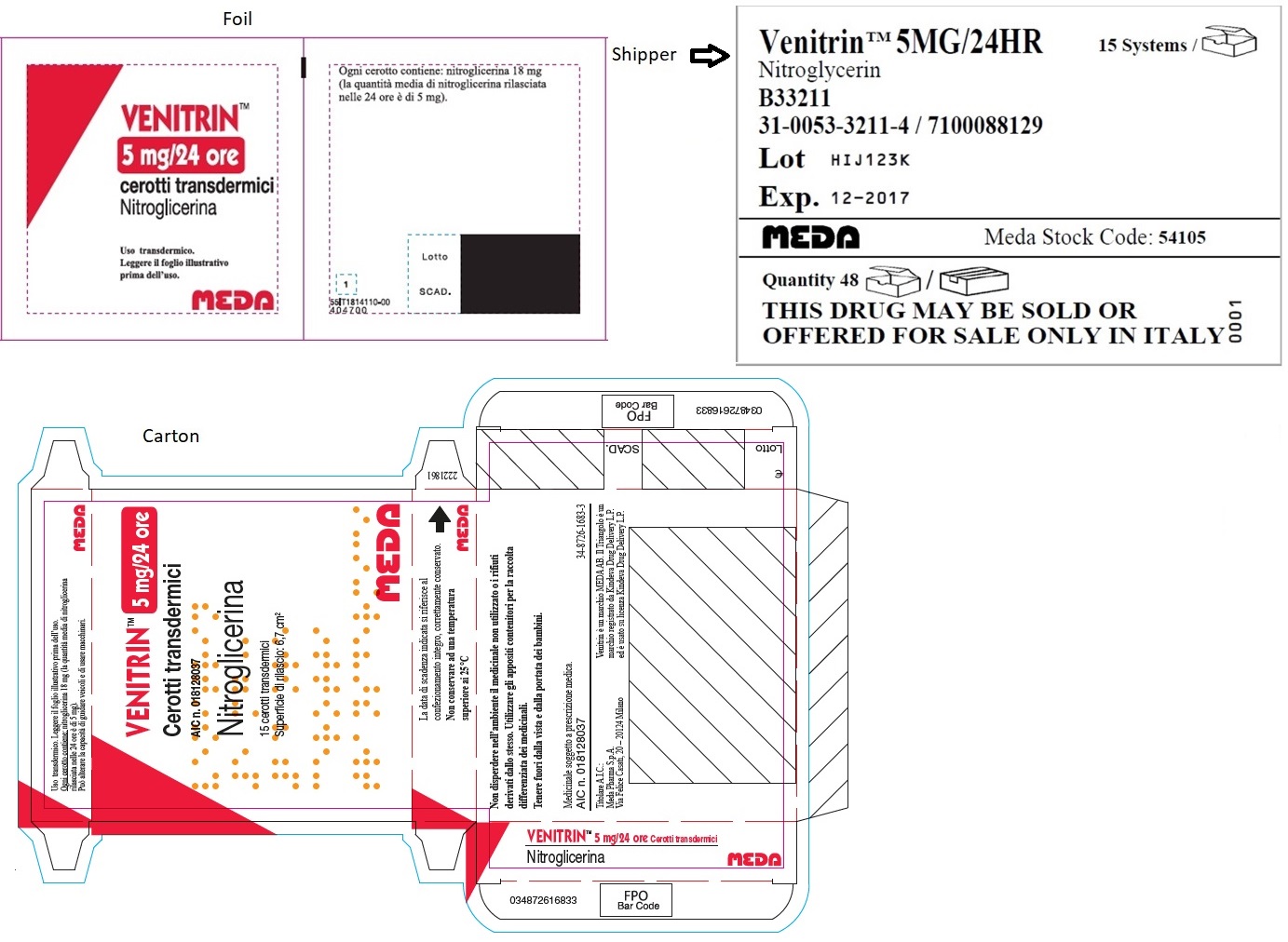
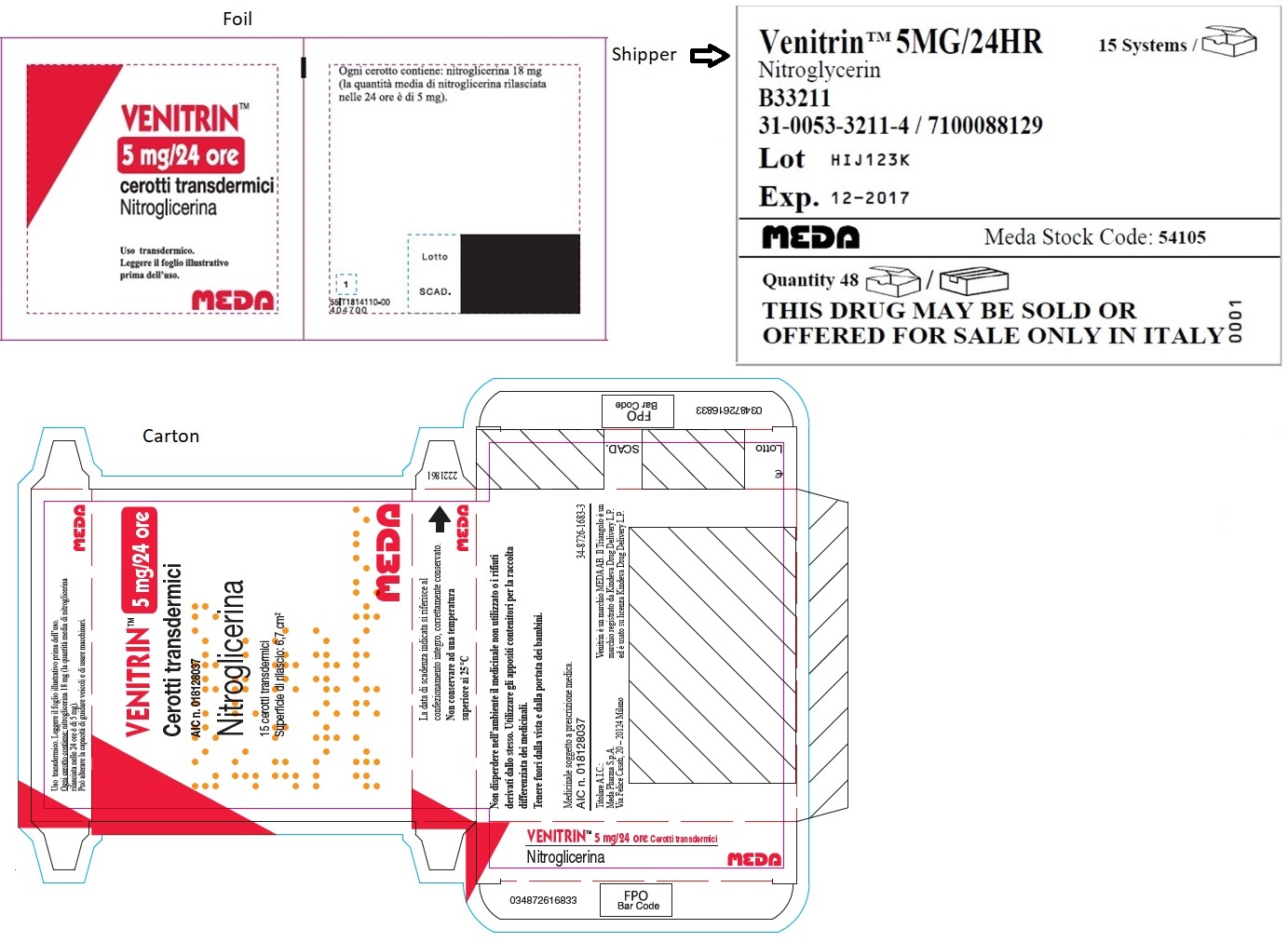
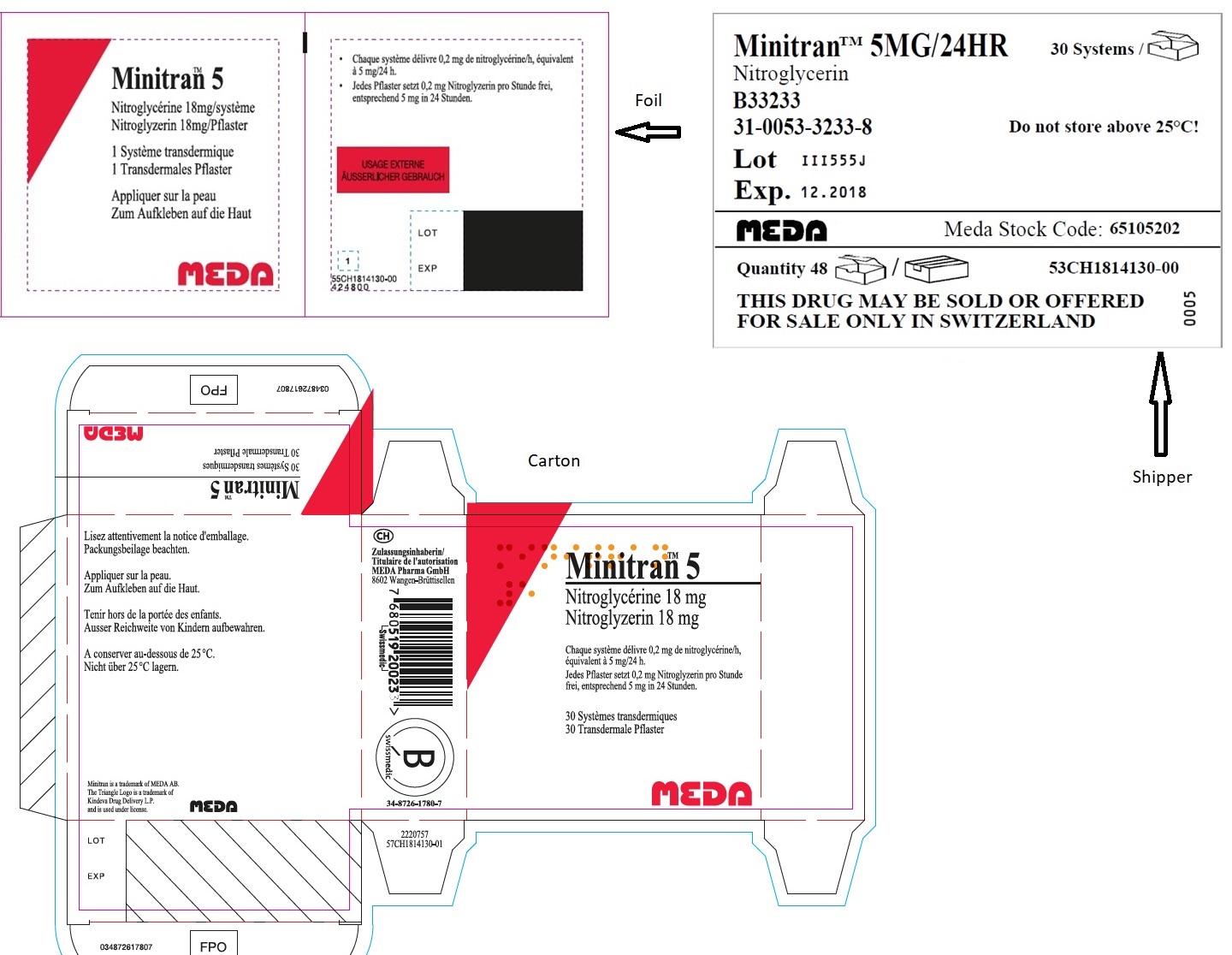
- MINITRAN™ 5 mg (Italy)
- VENITRAN™ 5 mg (Italy)
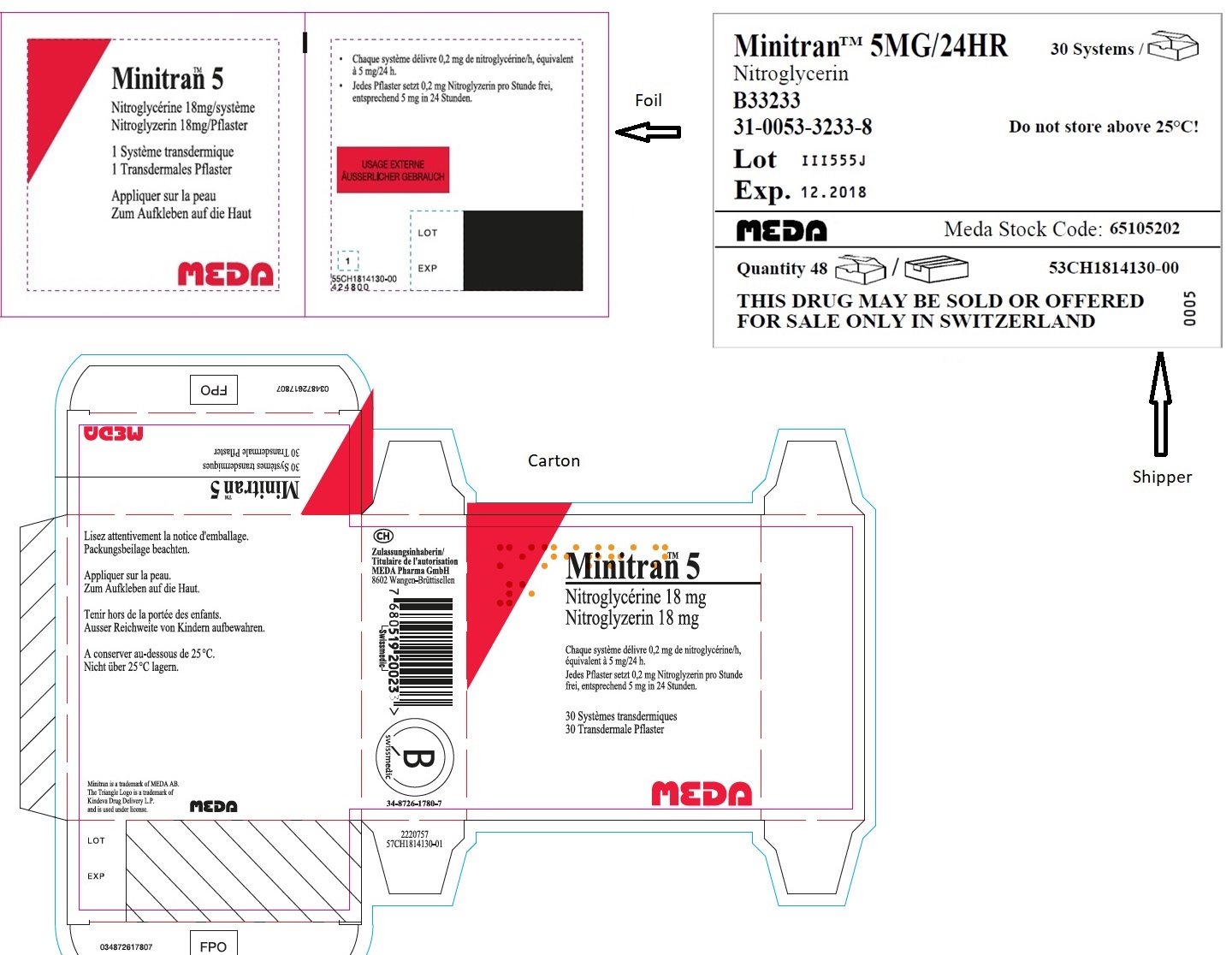
- MINITRAN™ 5 mg (Switzerland)
-
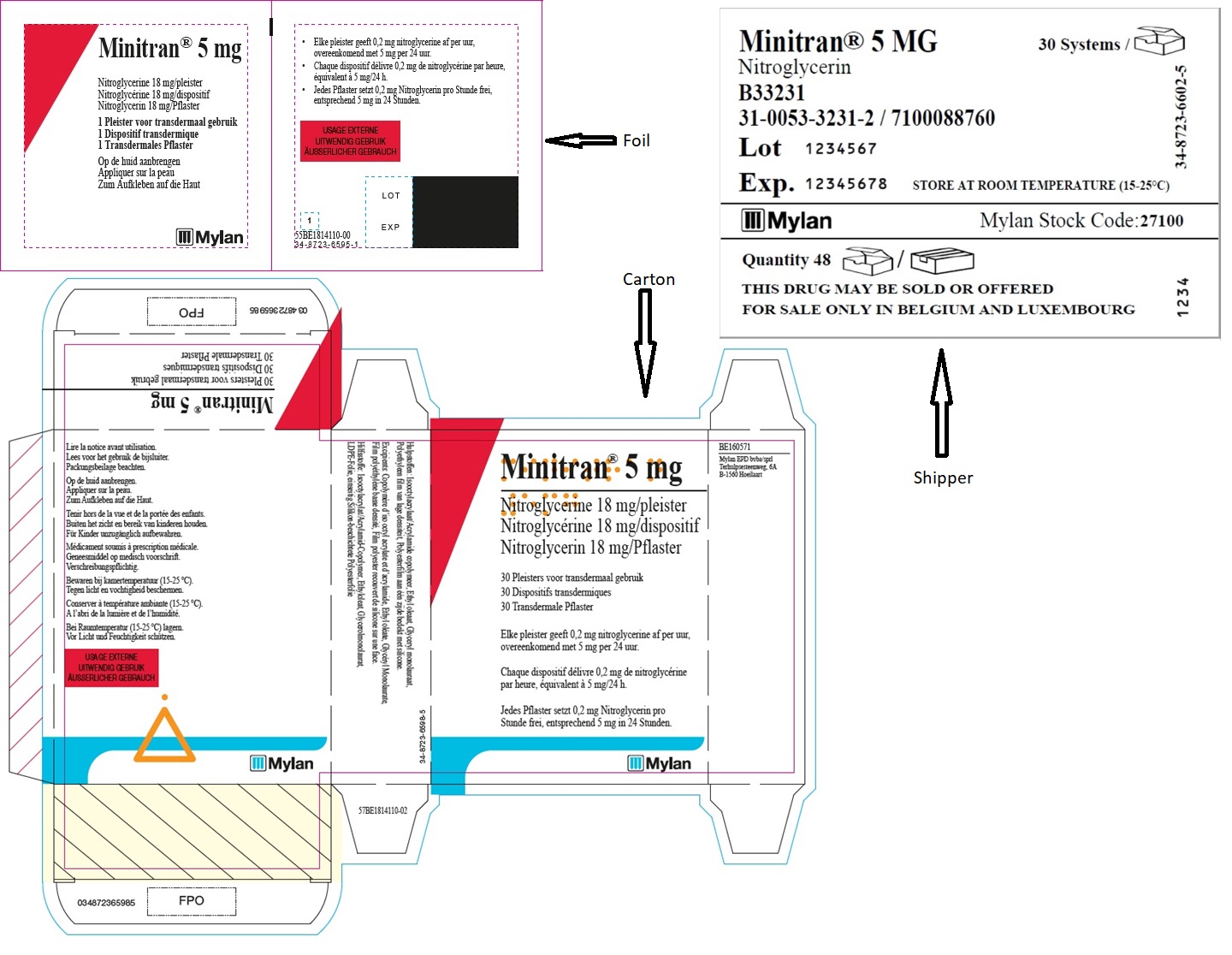
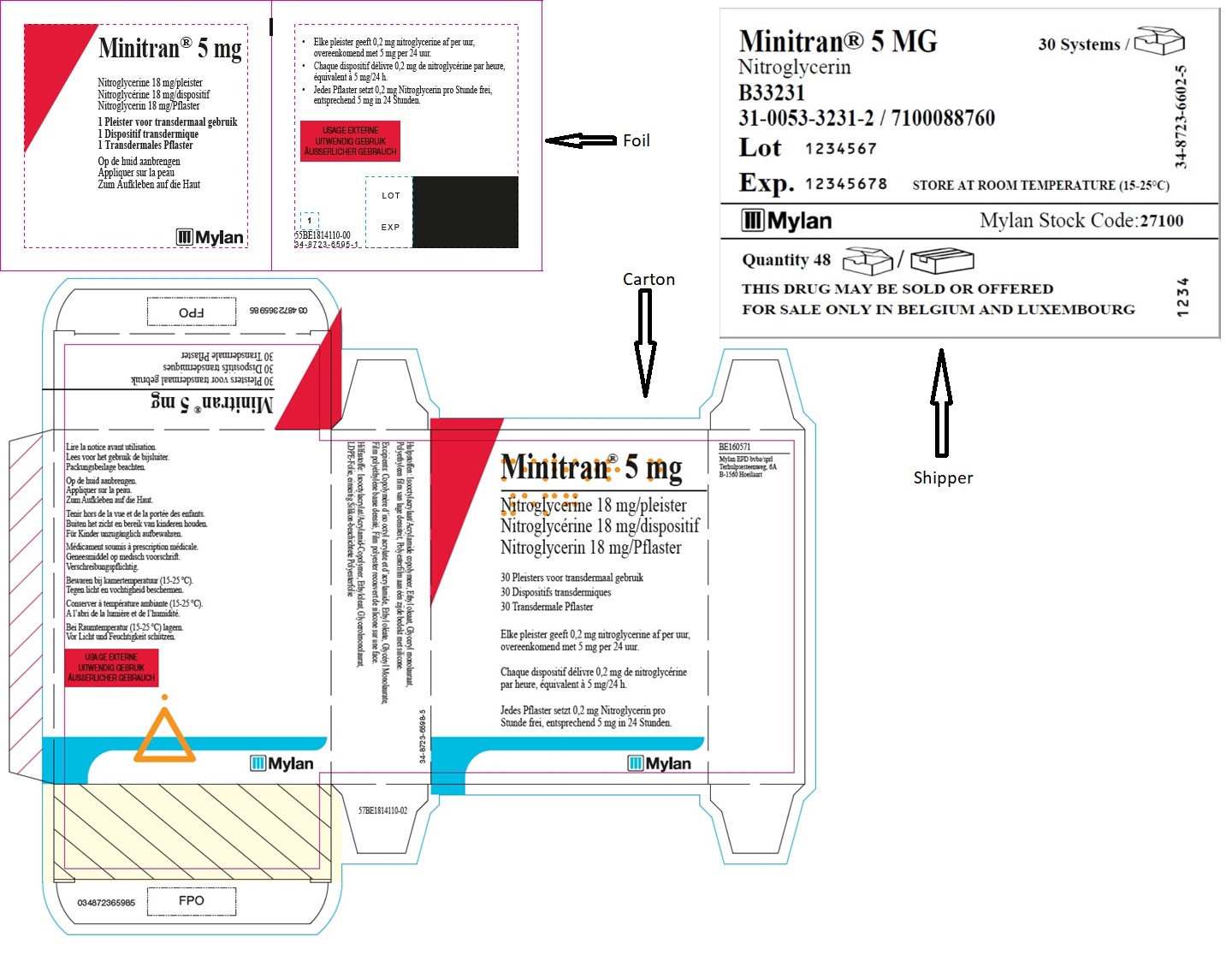
MINITRAN™ 5 mg (Belgium)
MINITRAN™ 5 mg
Nitroglycerine 18 mg/pleister
Nitroglycérine 18 mg/dispositif
Nitroglycerin 18 mg/pflaster
30 Pleisters voor transdermaal gebruik
30 Dispositifs transdermiques
30 Transdermale Pflaster
Elke pleister geeft 0,2 mg nitroglycerine af per uur,
overeenkomend met 5 mg per 24 uur.
Chaque dispositif délivre 0,23 mg de nitroglycérine
par heure, équivalent à 5 mg/24 h.
Jedes Pflaster setzt 0,2 mg Nitroglycerin pro
Stunde frei, entsprechend 5 mg in 24 Stunden.
MYLAN
-
MINITRAN™ 5 mg (Mexico)
MINITRAN™
Trinitrato de glicerilo
31-0053-3204-9
Lote: 999991A
Cad: MAR18
Reg. No. 074M91 SSA IV
Parche 18 mg
Cada parche libera 0.2 mg por hora. 5 mg/24 horas
Hecho en EUA por:
3M Drug Delivery Systems
Northridge CA, 91324, EUA
Importado y Distribuido por:
More Pharma Corporation S. de R.L. de C.V.
Blvd. Adolfo Lopez Mateos No. 314 PB, Col.
Tiacopac, C.P. 01049
Deleg. Alvaro Obregón
Ciudad de México, México
More Pharma
Pharmaceutical Company
Consérvese a temperatura ambiente a no mas de
30ºC y en lugar seco.
Quantity 5760

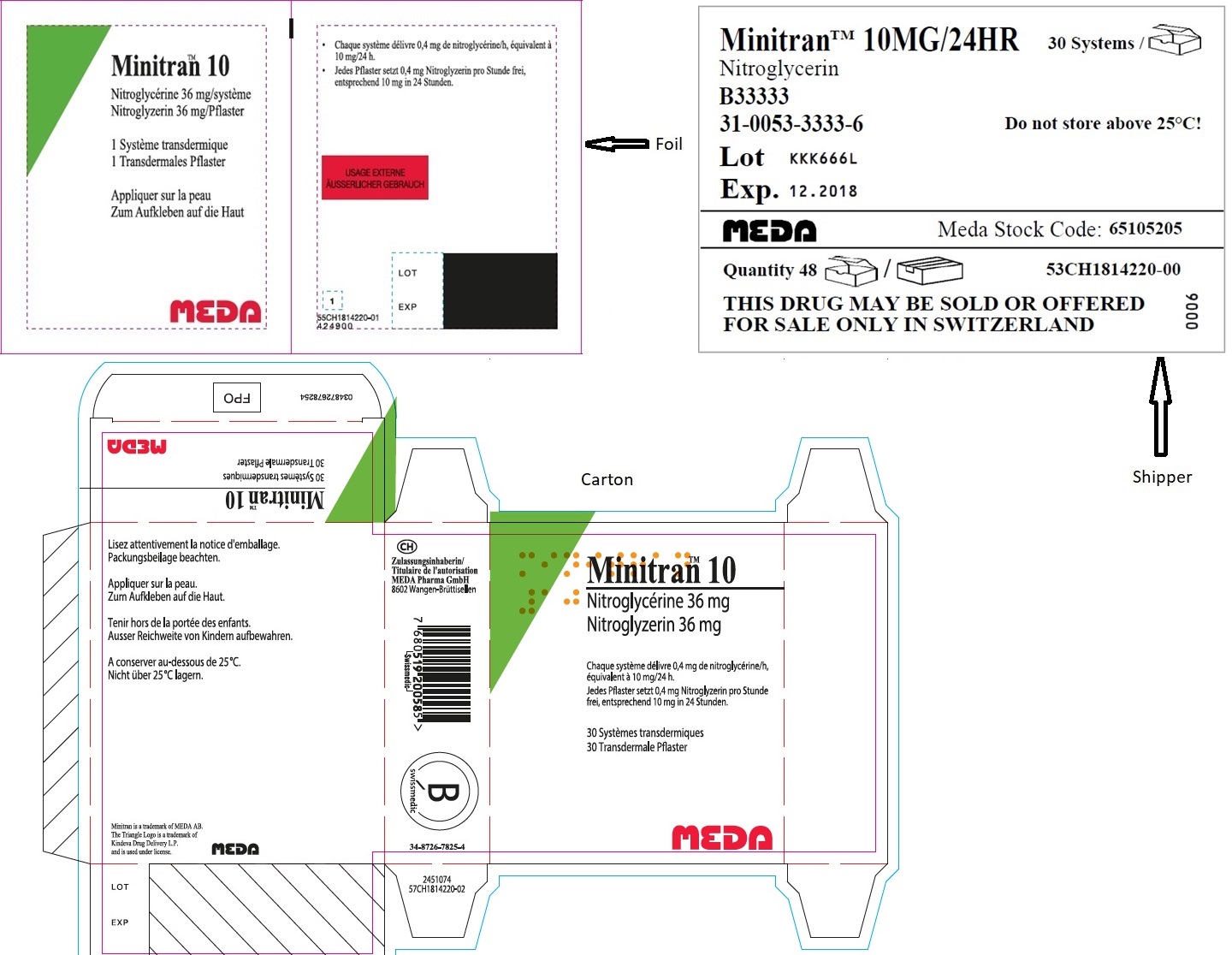
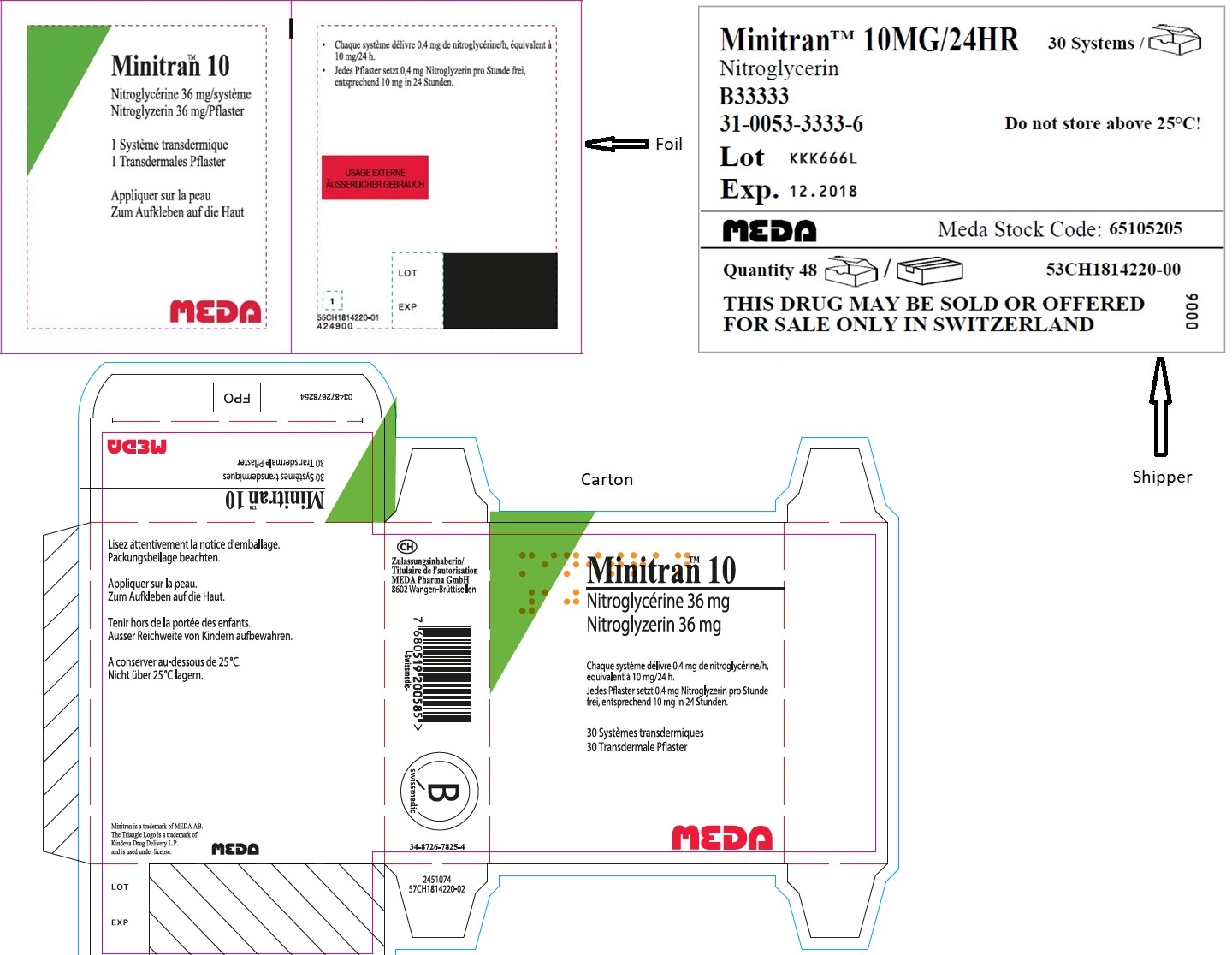
- MINITRAN™ 10 mg (Italy)
- VENITRAN™ 10 mg (Italy)
-
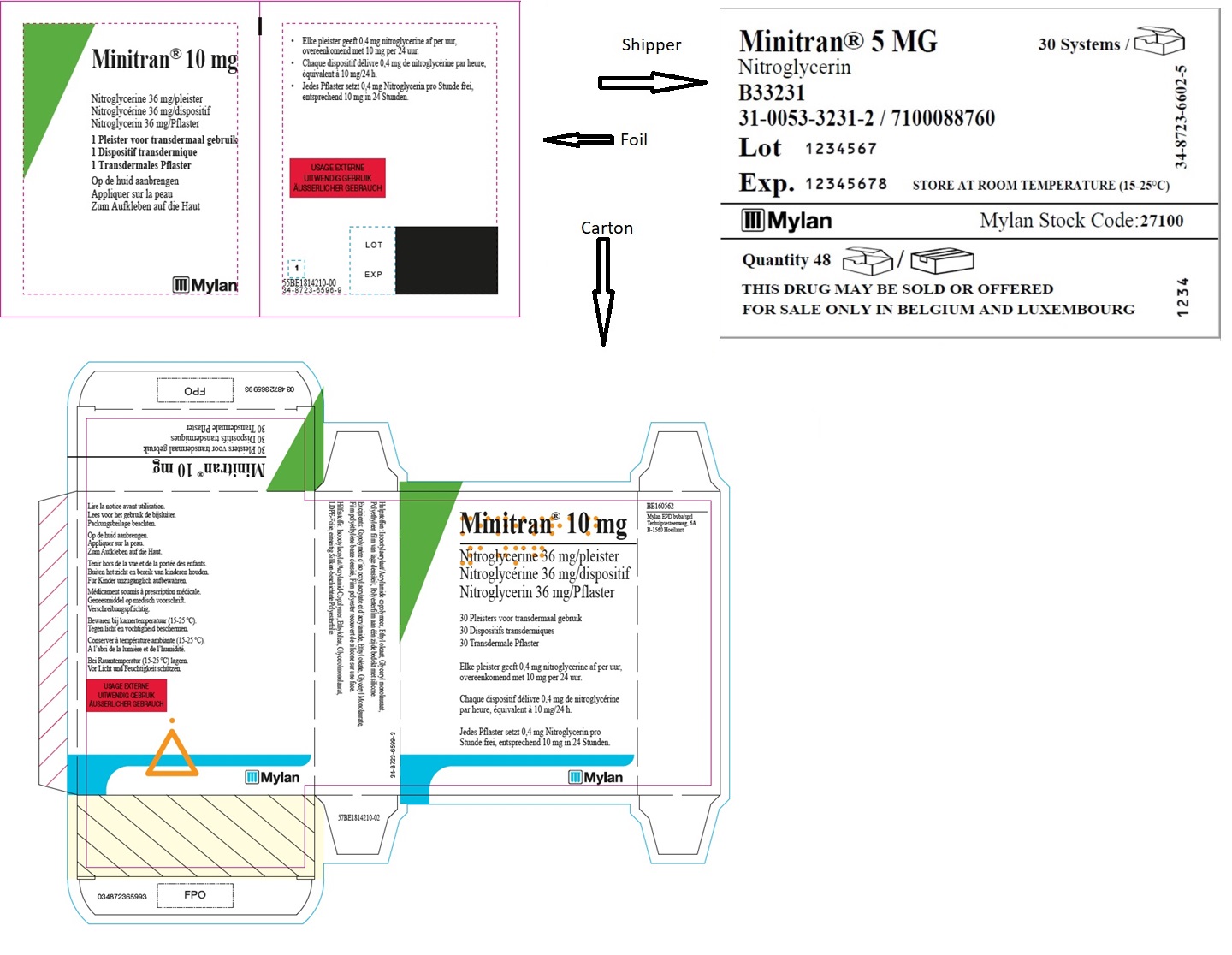
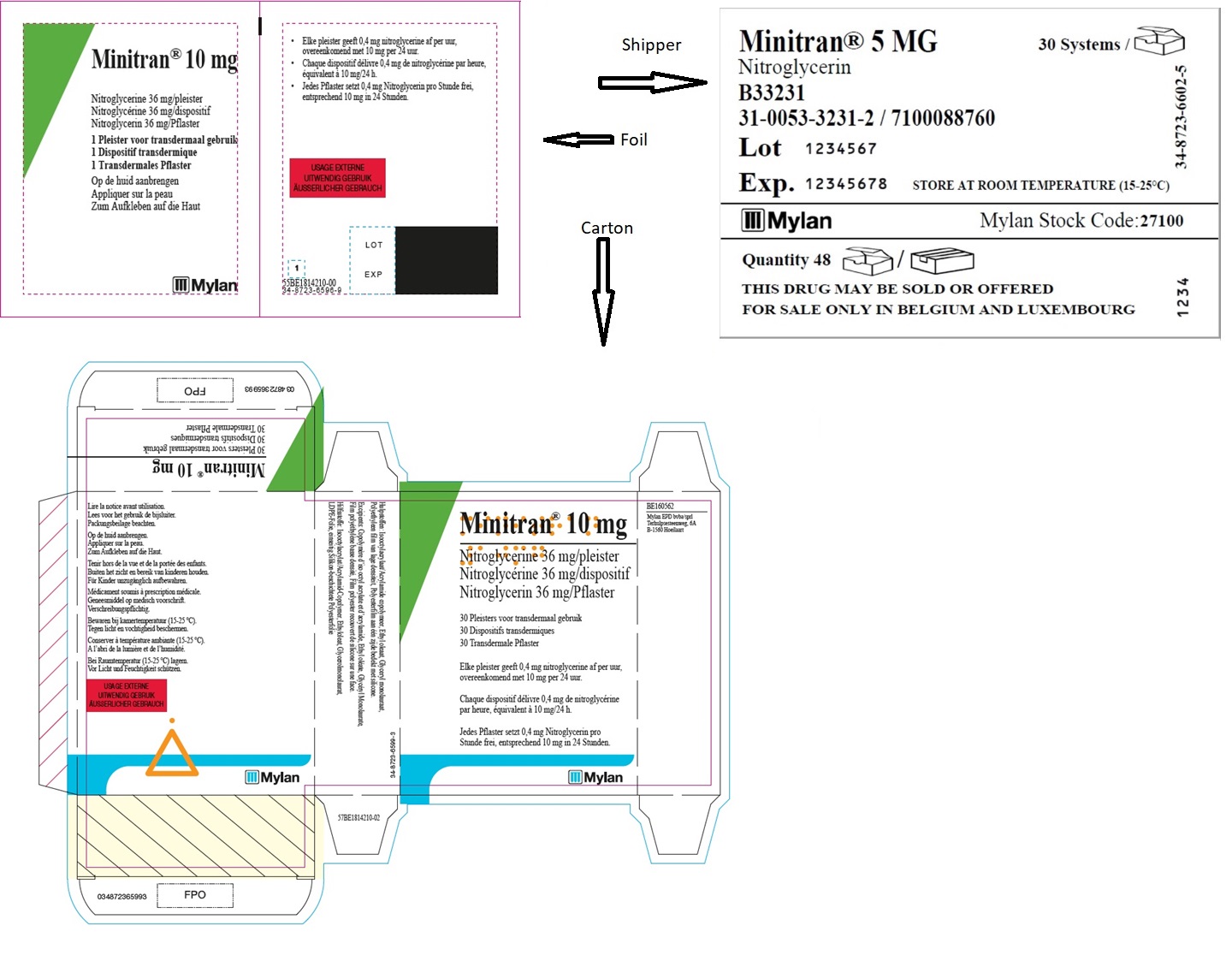
MINITRAN® 10 mg (Belgium)
MINITRAN® 10 mg
Nitroglycerine 36 mg/pleister
Nitroglycérine 36 mg/dispositif
Nitroglycerin 36 mg/pflaster
30 Pleisters voor transdermaal gebruik
30 Dispositifs transdermiques
30 Transdermale Pflaster
Elke pleister geeft 0,4 mg nitroglycerine af per uur,
overeenkomend met 10 mg per 24 uur.
Chaque dispositif délivre 0,4 mg de nitroglycérine
par heure, équivalent à 10mg/24 h.
Jedes Pflaster setzt 0,4 mg Nitroglycerin pro
Stunde frei, entsprechend 10 mg in 24 Stunden.
MYLAN
- MINITRAN™ 10 (Switzerland)
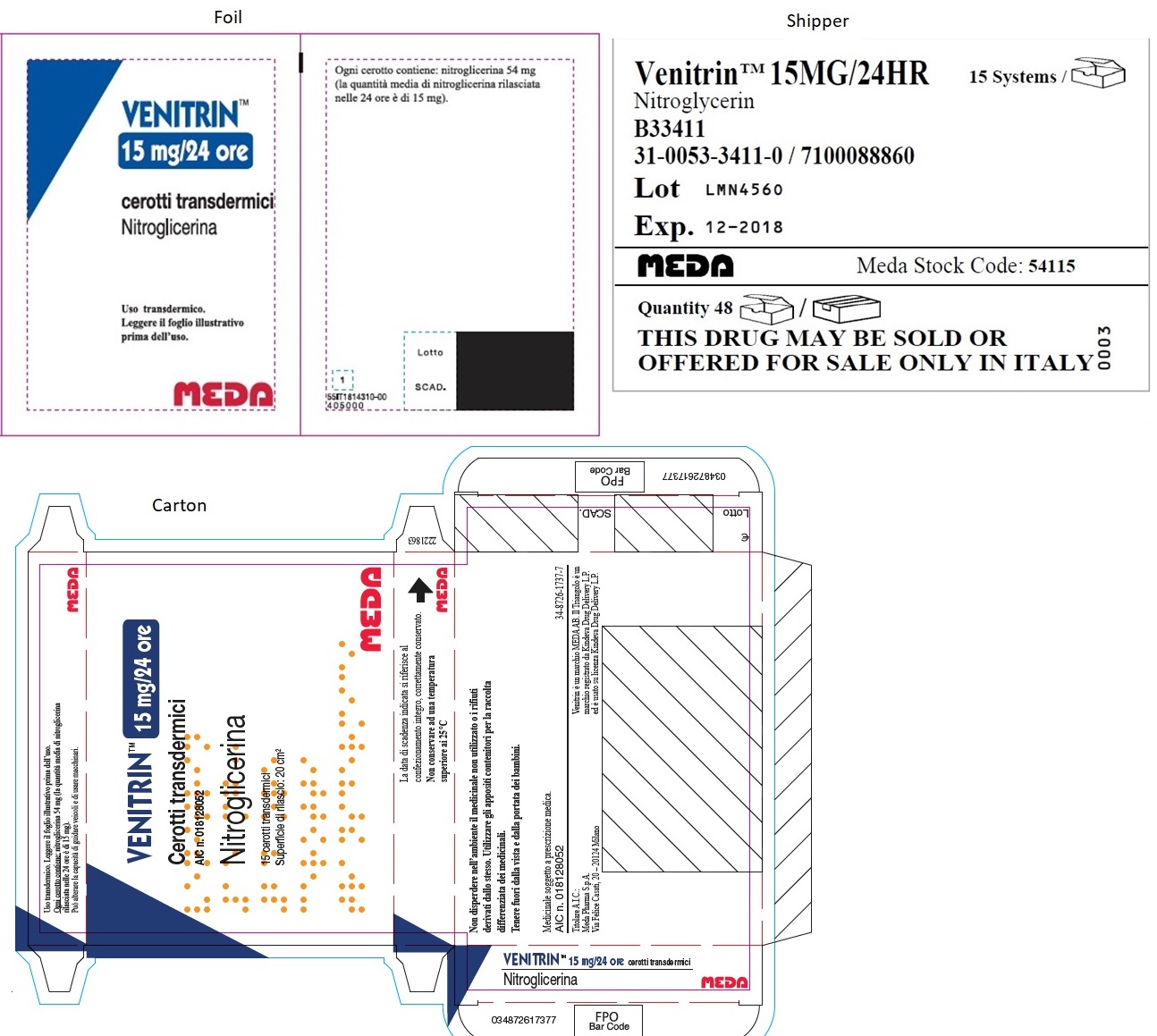
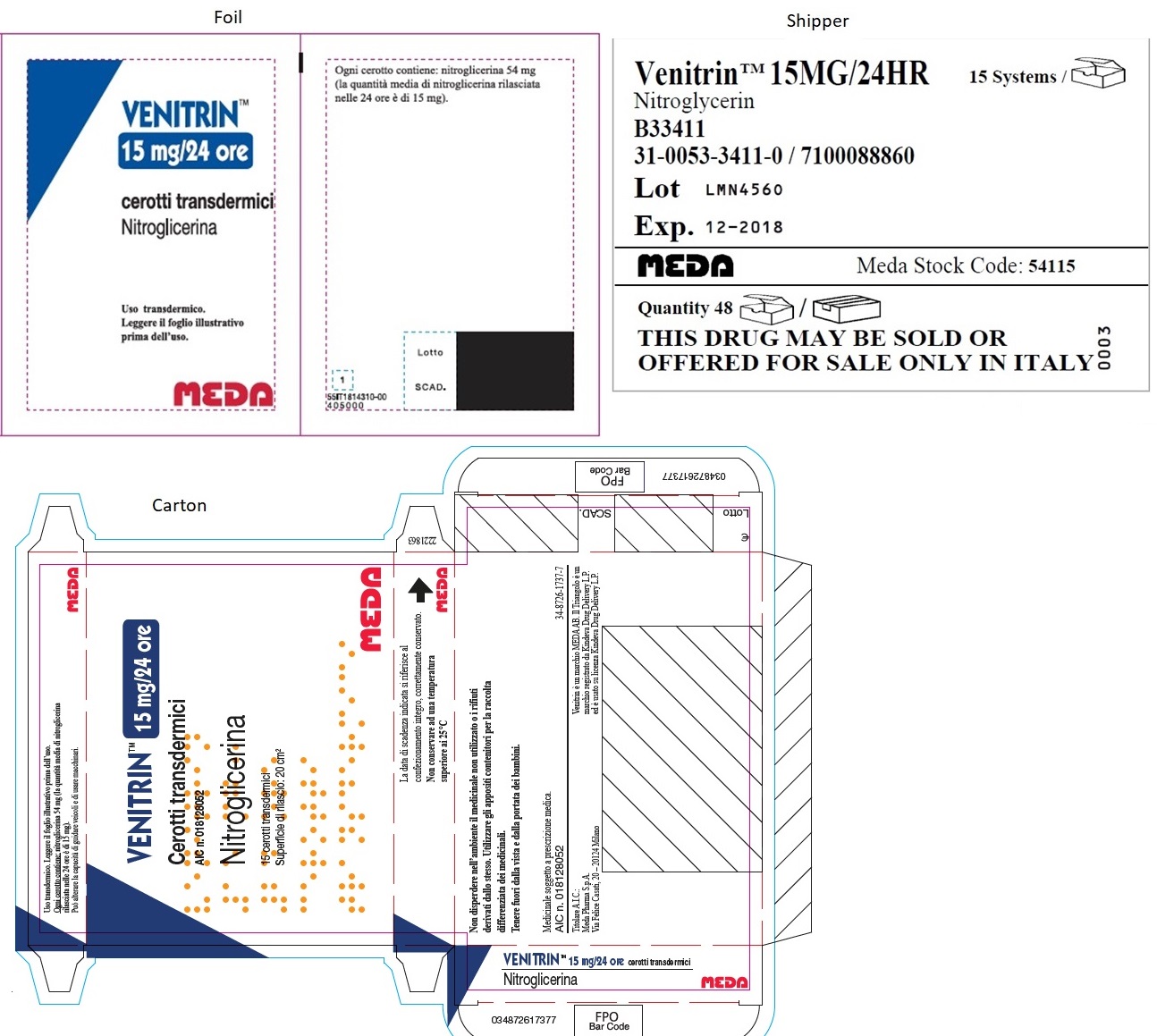
- MINITRAN™ 15 mg (Italy)
- VENITRAN™ 15 mg (Italy)
-
MINITRAN™ 15 mg (Belgium)
MINITRAN™ 15 mg
Nitroglycerine 54 mg/pleister
Nitroglycérine 54 mg/dispositif
Nitroglycerin 54 mg/pflaster
30 Pleisters voor transdermaal gebruik
30 Dispositifs transdermiques
30 Transdermale Pflaster
Elke pleister geeft 0,6 mg nitroglycerine af per uur,
overeenkomend met 15 mg per 24 uur.
Chaque dispositif délivre 0,6 mg de nitroglycérine
par heure, équivalent à 15 mg/24 h.
Jedes Pflaster setzt 0,6 mg Nitroglycerin pro
Stunde frei, entsprechend 15 mg in 24 Stunden.
MYLAN

-
MINITRAN™ 10 mg (Mexico)
MINITRAN™
Trinitrato de Glicerilo
Parche
36 mg
Cada Parche Libera 0.4 mg por hora
10 mg/24 horas
Lote/Lot: XXXXXX
Cad./Exp.: YYYXX
5760
Consérvese a temperatura
ambiente a no más de
30° yen en lugar seco.
Reg. No. 074M91 SSA IV
T M: Marca Registrada/Registered Trademark
More Pharma
Pharmaceutical Company
31-0053-3304-7
Distribuido Por:
More Pharma Corporation S. De R.L.. De C.V.
Av. Ejército Nacional No. 926
Col. Los morales, Seccion Palmas
C.P. 11540, Dele. Miguel Hidalgo,
D.F., México.
Hecho en E.U.A Por:
3M Drug Delivery Systems,
Northridge, Ca 91324 EUA

-
INGREDIENTS AND APPEARANCE
MINITRAN
nitroglycerin patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0089-0171 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 18 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0089-0171-02 30 in 1 CARTON; Type 0: Not a Combination Product 02/21/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 02/21/2012 MINITRAN
nitroglycerin patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0089-0172 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 36 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0089-0172-02 30 in 1 CARTON; Type 0: Not a Combination Product 02/21/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 02/21/2012 MINITRAN
nitroglycerin patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0089-0173 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROGLYCERIN (UNII: G59M7S0WS3) (NITROGLYCERIN - UNII:G59M7S0WS3) NITROGLYCERIN 54 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0089-0173-02 30 in 1 CARTON; Type 0: Not a Combination Product 02/21/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 02/21/2012 Labeler - Kindeva Drug Delivery L.P. (117492677) Establishment Name Address ID/FEI Business Operations Kindeva Drug Delivery L.P. 128688199 manufacture(0089-0171, 0089-0172, 0089-0173)