Label: UP AND UP CHILDRENS ALLERGY MELTS- diphenhydramine hcl tablet, chewable
- NDC Code(s): 11673-012-18, 11673-012-48
- Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
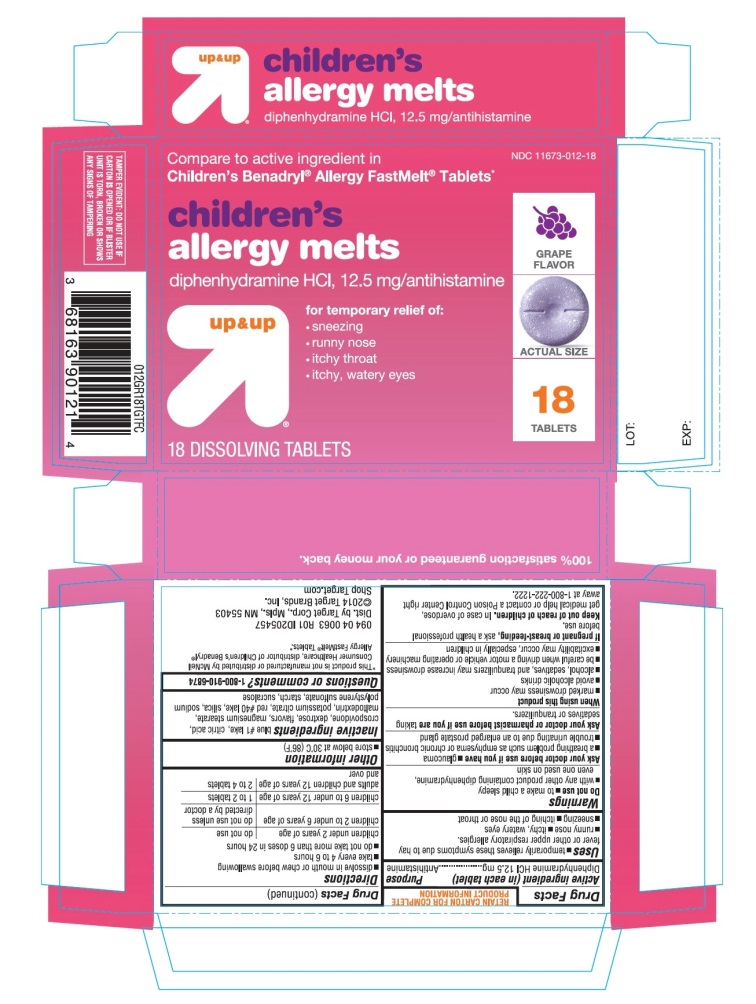
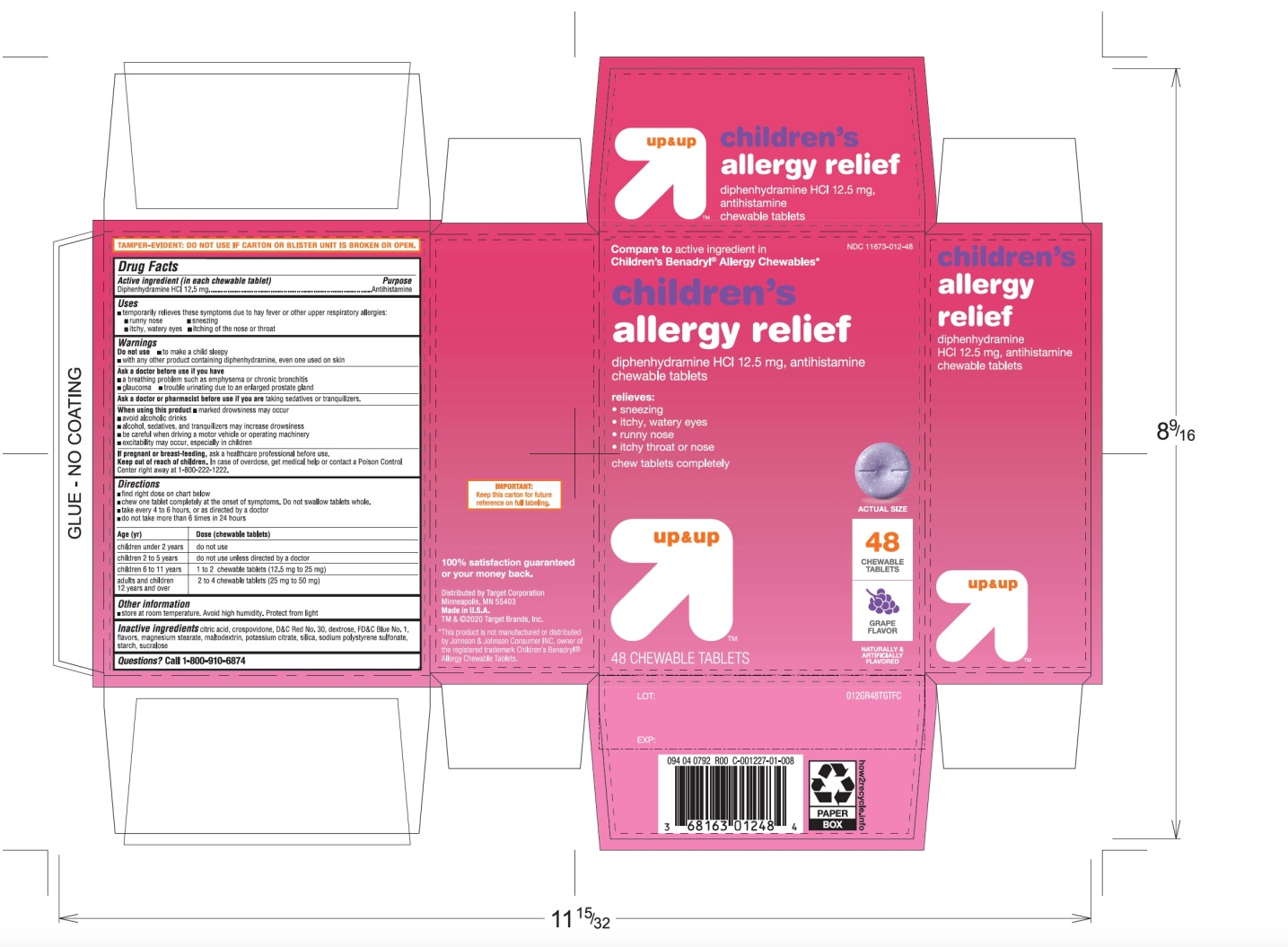
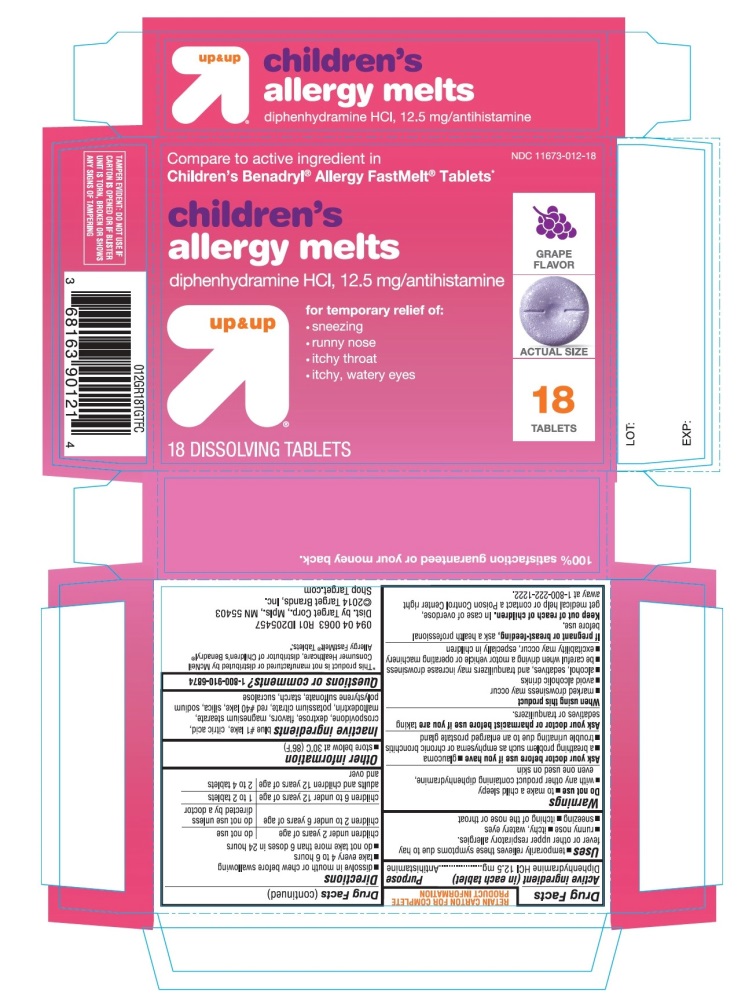
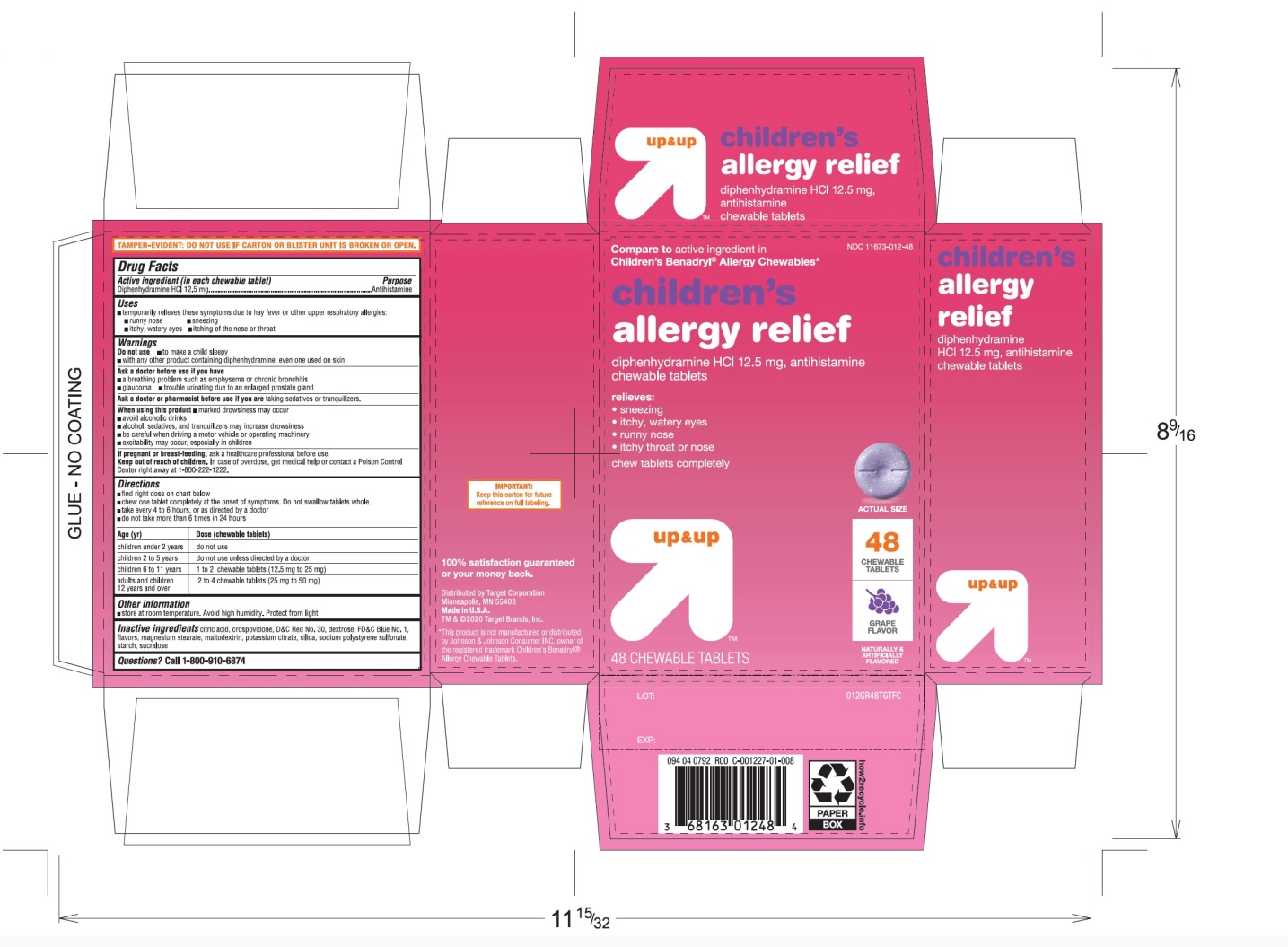
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- ▪
- to make a child sleepy
- ▪
- with any other product containing diphenhydramine, even one used on skin
Ask your doctor before use if you have
- ▪
- a breathing problem such as emphysema or chronic bronchitis
- ▪
- glaucoma
- ▪
- trouble urinating due to an enlarged prostate gland
-
Directions
- ▪
- find right dose on chart below
- ▪
- Chew one tablet completely at the onset of symptoms. Do not swallow tablets whole.
- ▪
- take every 4 to 6 hours or as directed by a doctor
- ▪
- do not take more than 6 times in 24 hours
children under 2 years
do not use
children 2 to 5 years
do not use unless directed by a doctor
children 6 to 11 years of age
1 to 2 chewable tablets (12.5 mg to 25 mg)
adults and children 12 years of age and over
2 to 4 tablets (25 mg to 50 mg)
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Compare to active ingredient in Children's Benadryl® Allergy Chewables*
Children’s Allergy relief
Diphenhydramine HCl, 12.5 mg/ AntihistamineFor relief of:
- •
- sneezing
- •
- runny nose
- •
- itchy throat
- •
- itchy, watery eyes
4-6 Hours/Dose
up & up
Grape Flavor
Naturally and Artificially Flavored
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Johnson & Johnson Consumer INC, owner of the registered trademark Children's Benadryl® Allergy Chewable Tablets*.
Distributed by Target CorporationMinneapolis, MN 55403
Made in USA
TM & ©2020 Target Brands, Inc.100% satisfaction guaranteed or your money back.
Children’s Allergy Grape Flavor 18 Chewable Tablets
-
INGREDIENTS AND APPEARANCE
UP AND UP CHILDRENS ALLERGY MELTS
diphenhydramine hcl tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-012 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) D&C RED NO. 30 (UNII: 2S42T2808B) FD&C RED NO. 40 (UNII: WZB9127XOA) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) POTASSIUM CITRATE (UNII: EE90ONI6FF) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) STARCH, CORN (UNII: O8232NY3SJ) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color PURPLE Score 2 pieces Shape ROUND Size 16mm Flavor GRAPE Imprint Code RP012 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-012-18 3 in 1 CARTON 11/10/2015 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:11673-012-48 4 in 1 CARTON 10/30/2019 2 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 11/01/2015 Labeler - Target Corporation (006961700)