Label: HAVRIX- hepatitis a vaccine injection, suspension
- NDC Code(s): 58160-825-43, 58160-825-52, 58160-826-43, 58160-826-52
- Packager: GlaxoSmithKline Biologicals SA
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HAVRIX safely and effectively. See full prescribing information for HAVRIX.
HAVRIX (Hepatitis A Vaccine) injectable suspension, for intramuscular use
Initial U.S. Approval: 1995
RECENT MAJOR CHANGES
Warnings and Precautions, Latex (5.1) - Removed
10/2023
INDICATIONS AND USAGE
HAVRIX is a vaccine indicated for active immunization against disease caused by hepatitis A virus (HAV). HAVRIX is approved for use in persons 12 months of age and older. Primary immunization should be administered at least 2 weeks prior to expected exposure to HAV. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis A-containing vaccine, or to any component of HAVRIX, including neomycin. (4)
WARNINGS AND PRECAUTIONS
- Syncope (fainting) can occur in association with administration of injectable vaccines, including HAVRIX. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope. (5.1)
ADVERSE REACTIONS
- •
- In studies of adults and children 2 years of age and older, the most common solicited adverse reactions were injection-site soreness (56% of adults and 21% of children) and headache (14% of adults and less than 9% of children). (6.1)
- •
- In studies of children 11 to 25 months of age, the most frequently reported solicited local reactions were pain (32%) and redness (29%). Common solicited general adverse reactions were irritability (42%), drowsiness (28%), and loss of appetite (28%). (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
DRUG INTERACTIONS
Do not mix HAVRIX with any other vaccine or product in the same syringe. (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Administration
2.2 Administration
2.3 Recommended Dose and Schedule
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Syncope
5.2 Preventing and Managing Allergic Vaccine Reactions
5.3 Altered Immunocompetence
5.4 Limitations of Vaccine Effectiveness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Vaccines and Immune Globulin
7.2 Immunosuppressive Therapies
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Pediatric Effectiveness Studies
14.2 Immunogenicity in Children and Adolescents
14.3 Immunogenicity in Adults
14.4 Duration of Immunity
14.5 Immune Response to Concomitantly Administered Vaccines
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Administration
Shake well before use. With thorough agitation, HAVRIX is a homogeneous, turbid, white suspension. Do not administer if it appears otherwise. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If either of these conditions exists, the vaccine should not be administered. Attach a sterile needle to the prefilled syringe and administer intramuscularly.
2.2 Administration
HAVRIX should be administered by intramuscular injection only. HAVRIX should not be administered in the gluteal region; such injections may result in suboptimal response.
Do not administer this product intravenously, intradermally, or subcutaneously.
2.3 Recommended Dose and Schedule
Children and Adolescents (aged 12 months through 18 years)
Primary immunization for children and adolescents consists of a single 0.5-mL dose and a 0.5-mL booster dose administered anytime between 6 and 12 months later. The preferred sites for intramuscular injections are the anterolateral aspect of the thigh in young children or the deltoid muscle of the upper arm in older children.
Adults (aged 19 years and older)
Primary immunization for adults consists of a single 1-mL dose and a 1-mL booster dose administered anytime between 6 and 12 months later. In adults, the injection should be given in the deltoid region.
-
3 DOSAGE FORMS AND STRENGTHS
Suspension for injection available in the following presentations:
- •
- 0.5-mL single-dose prefilled TIP-LOK syringes.
- •
- 1-mL single-dose prefilled TIP-LOK syringes. [See How Supplied/Storage and Handling (16).]
-
4 CONTRAINDICATIONS
Severe allergic reaction (e.g., anaphylaxis) after a previous dose of any hepatitis A-containing vaccine, or to any component of HAVRIX, including neomycin, is a contraindication to administration of HAVRIX [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Syncope
Syncope (fainting) can occur in association with administration of injectable vaccines, including HAVRIX. Syncope can be accompanied by transient neurological signs such as visual disturbance, paresthesia, and tonic-clonic limb movements. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope.
5.2 Preventing and Managing Allergic Vaccine Reactions
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of the vaccine [see Contraindications (4)].
5.3 Altered Immunocompetence
Immunocompromised persons may have a diminished immune response to HAVRIX, including individuals receiving immunosuppressant therapy.
5.4 Limitations of Vaccine Effectiveness
Hepatitis A virus has a relatively long incubation period (15 to 50 days). HAVRIX may not prevent hepatitis A infection in individuals who have an unrecognized hepatitis A infection at the time of vaccination. Additionally, vaccination with HAVRIX may not protect all individuals.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of HAVRIX has been evaluated in 61 clinical trials involving approximately 37,000 individuals receiving doses of 360 EL.U. (n = 21,928 in 3- or 4-dose schedule), 720 EL.U. (n = 12,274 in 2- or 3-dose schedule), or 1440 EL.U. (n = 2,782 in 2- or 3-dose schedule).
Of solicited adverse reactions in clinical trials of adults, who received HAVRIX 1440 EL.U., and children (aged 2 years and older), who received either HAVRIX 360 EL.U. or 720 EL.U., the most frequently reported was injection-site soreness (56% of adults and 21% of children); less than 0.5% of soreness was reported as severe. Headache was reported by 14% of adults and less than 9% of children. Other solicited and unsolicited reactions occurring during clinical trials are listed below.
Incidence 1% to 10% of Injections
Metabolism and Nutrition Disorders: Anorexia.
Gastrointestinal Disorders: Nausea.
General Disorders and Administration Site Conditions: Fatigue; fever >99.5°F (37.5°C); induration, redness, and swelling of the injection site; malaise.
Incidence <1% of Injections
Infections and Infestations: Pharyngitis, upper respiratory tract infections.
Blood and Lymphatic System Disorders: Lymphadenopathy.
Psychiatric Disorders: Insomnia.
Nervous System Disorders: Dysgeusia, hypertonia.
Eye Disorders: Photophobia.
Ear and Labyrinth Disorders: Vertigo.
Gastrointestinal Disorders: Abdominal pain, diarrhea, vomiting.
Skin and Subcutaneous Tissue Disorders: Pruritus, rash, urticaria.
Musculoskeletal and Connective Tissue Disorders: Arthralgia, myalgia.
General Disorders and Administration Site Conditions: Injection site hematoma.
Investigations: Creatine phosphokinase increased.
Coadministration Studies of HAVRIX in Children Aged 11 to 25 Months
In 4 studies, 3,152 children aged 11 to 25 months received at least 1 dose of HAVRIX 720 EL.U. administered alone or concomitantly with other routine childhood vaccinations [see Clinical Studies (14.2, 14.5)]. The studies included HAV 210 (N = 1,084), HAV 232 (N = 394), HAV 220 (N = 433), and HAV 231 (N = 1,241).
In the largest of these studies (HAV 231) conducted in the U.S., 1,241 children aged 15 months were randomized to receive: Group 1) HAVRIX alone; Group 2) HAVRIX concomitantly with measles, mumps, and rubella (MMR) vaccine (manufactured by Merck and Co.) and varicella vaccine (manufactured by Merck and Co.); or Group 3) MMR and varicella vaccines. Subjects in Group 3 who received MMR and varicella vaccines received the first dose of HAVRIX 42 days later. A second dose of HAVRIX was administered to all subjects 6 to 9 months after the first dose of HAVRIX. Solicited local adverse reactions and general events were recorded by parents/guardians on diary cards for 4 days (Days 0 to 3) after vaccination. Unsolicited adverse events were recorded on the diary card for 31 days after vaccination. Telephone follow-up was conducted 6 months after the last vaccination to inquire about serious adverse events, new onset chronic illnesses, and medically significant events. A total of 1,035 children completed the 6-month follow-up. Among subjects in all groups combined, 53% were male; 69% of subjects were White, 16% were Hispanic, 9% were Black, and 6% were other racial/ethnic groups.
Percentages of subjects with solicited local adverse reactions and general adverse reactions following HAVRIX administered alone (Group 1) or concomitantly with MMR and varicella vaccines (Group 2) are presented in Table 1. The solicited adverse reactions from the 3 additional coadministration studies conducted with HAVRIX were comparable to those from Study HAV 231.
Table 1. Solicited Local Adverse Reactions and General Adverse Reactions Occurring within 4 Days of Vaccinationa in Children Aged 15 to 24 Months with HAVRIX Administered Alone or Concomitantly with MMR and Varicella Vaccines (TVC) Total vaccinated cohort (TVC) = all subjects who received at least 1 dose of vaccine. n = Number of subjects who received at least 1 dose of vaccine and for whom diary card information was available. Grade 3: Drowsiness defined as prevented normal daily activities; irritability/fussiness defined as crying that could not be comforted/prevented normal daily activities; loss of appetite defined as no eating at all. a Within 4 days of vaccination defined as day of vaccination and the next 3 days. b MMR = Measles, mumps, and rubella vaccine; V = Varicella vaccine. Group 1
HAVRIX
Dose 1
%
Group 2
HAVRIX+
MMR+Vb
Dose 1
%
Group 1
HAVRIX
Dose 2
%
Group 2
HAVRIX
Dose 2
%
Local (at injection site for HAVRIX)
n
298
411
272
373
Pain, any
24
24
24
30
Redness, any
20
20
23
24
Swelling, any
9
10
10
10
General
n
300
417
271
375
Irritability, any
33
44
31
27
Irritability, Grade 3
0
2
2
0
Drowsiness, any
22
35
21
21
Drowsiness, Grade 3
1
2
1
0
Loss of appetite, any
18
26
20
21
Loss of appetite, Grade 3
1
1
0
0
Fever ≥100.6°F (38.1°C)
3
5
3
3
Fever ≥101.5°F (38.6°C)
2
3
2
2
Fever ≥102.4°F (39.1°C)
1
1
0
1
Serious Adverse Events in Children Aged 11 to 25 Months: Among these 4 studies, 0.9% (29/3,152) of subjects reported a serious adverse event within the 31-day period following vaccination with HAVRIX. Among subjects administered HAVRIX alone 1.0% (13/1,332) reported a serious adverse event. Among subjects who received HAVRIX concomitantly with other childhood vaccines, 0.9% (8/909) reported a serious adverse event. In these 4 studies, there were 4 reports of seizure within 31 days post-vaccination: these occurred 2, 9, and 27 days following the first dose of HAVRIX administered alone and 12 days following the second dose of HAVRIX. In 1 subject who received INFANRIX (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed) and Hib conjugate vaccine followed by HAVRIX 6 weeks later, bronchial hyperreactivity and respiratory distress were reported on the day of administration of HAVRIX alone.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of HAVRIX. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the vaccine.
Infections and Infestations
Rhinitis.
Blood and Lymphatic System Disorders
Thrombocytopenia.
Immune System Disorders
Anaphylactic reaction, anaphylactoid reaction, serum sickness–like syndrome.
Nervous System Disorders
Convulsion, dizziness, encephalopathy, Guillain-Barré syndrome, hypoesthesia, multiple sclerosis, myelitis, neuropathy, paresthesia, somnolence, syncope.
Vascular Disorders
Vasculitis.
Respiratory, Thoracic, and Mediastinal Disorders
Dyspnea.
Hepatobiliary Disorders
Hepatitis, jaundice.
Skin and Subcutaneous Tissue Disorders
Angioedema, erythema multiforme, hyperhidrosis.
Congenital, Familial, and Genetic Disorders
Congenital anomaly.
Musculoskeletal and Connective Tissue Disorders
Musculoskeletal stiffness.
General Disorders and Administration Site Conditions
Chills, influenza-like symptoms, injection site reaction, local swelling.
-
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Vaccines and Immune Globulin
In clinical studies HAVRIX was administered concomitantly with the following vaccines [see Adverse Reactions (6.1), Clinical Studies (14.5)].
- •
- INFANRIX (DTaP);
- •
- Hib conjugate vaccine;
- •
- pneumococcal 7-valent conjugate vaccine;
- •
- MMR vaccine;
- •
- varicella vaccine.
HAVRIX may be administered concomitantly with immune globulin.
When concomitant administration of other vaccines or immune globulin is required, they should be given with different syringes and at different injection sites. Do not mix HAVRIX with any other vaccine or product in the same syringe.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
There are no adequate and well-controlled studies of HAVRIX in pregnant women in the U.S. Available data do not suggest an increased risk of major birth defects and miscarriage in women who received HAVRIX during pregnancy (see Data).
There are no animal studies with HAVRIX to inform use during pregnancy.
Data
Human Data: In pre- and post-licensure clinical studies of HAVRIX, 175 pregnant women (177 outcomes, including two sets of twins) were inadvertently administered HAVRIX following their last menstrual period. After excluding ectopic pregnancies (n = 2), molar pregnancies (n = 1), elective terminations (n = 22, including one of a fetus with a birth defect), those that were lost to follow-up (n = 9), and those with an unknown exposure timing (n = 5), there were 138 known pregnancy outcomes with exposure during the first or second trimester. Of these, miscarriage was reported in 11% of pregnancies exposed prior to 20 weeks gestation (15/136) and major birth defects were reported in 3.3% (4/123) of live births. The rates of miscarriage and major birth defects were consistent with estimated background rates.
8.2 Lactation
Risk Summary
There is no information regarding the presence of HAVRIX in human milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for HAVRIX and any potential adverse effects on the breastfed child from HAVRIX or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
The safety and effectiveness of HAVRIX, doses of 360 EL.U. or 720 EL.U., have been evaluated in more than 22,000 subjects aged 1 to 18 years.
The safety and effectiveness of HAVRIX have not been established in subjects younger than 12 months.
8.5 Geriatric Use
Clinical studies of HAVRIX did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in overall safety between these subjects and younger adult subjects.
8.6 Hepatic Impairment
Subjects with chronic liver disease had a lower antibody response to HAVRIX than healthy subjects [see Clinical Studies (14.3)].
-
11 DESCRIPTION
HAVRIX (Hepatitis A Vaccine) is a sterile suspension of inactivated virus for intramuscular administration. The virus (strain HM175) is propagated in MRC-5 human diploid cells. After removal of the cell culture medium, the cells are lysed to form a suspension. This suspension is purified through ultrafiltration and gel permeation chromatography procedures. Treatment of this lysate with formalin ensures viral inactivation. Viral antigen activity is referenced to a standard using an enzyme linked immunosorbent assay (ELISA), and is therefore expressed in terms of ELISA Units (EL.U.).
Each 1-mL adult dose of vaccine contains 1440 EL.U. of viral antigen, adsorbed on 0.5 mg of aluminum as aluminum hydroxide.
Each 0.5-mL pediatric dose of vaccine contains 720 EL.U. of viral antigen, adsorbed onto 0.25 mg of aluminum as aluminum hydroxide.
HAVRIX contains the following excipients: Amino acid supplement (0.3% w/v) in a phosphate-buffered saline solution and polysorbate 20 (0.05 mg/mL). From the manufacturing process, HAVRIX also contains residual MRC-5 cellular proteins (not more than 5 mcg/mL), formalin (not more than 0.1 mg/mL), and neomycin sulfate (not more than 40 ng/mL), an aminoglycoside antibiotic included in the cell growth media.
HAVRIX is formulated without preservatives.
HAVRIX is available in prefilled syringes. The tip cap and rubber plunger stopper of the prefilled syringe are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The hepatitis A virus belongs to the picornavirus family. It is 1 of several hepatitis viruses that cause systemic disease with pathology in the liver.
The incubation period for hepatitis A averages 28 days (range: 15 to 50 days).1 The course of hepatitis A infection is extremely variable, ranging from asymptomatic infection to icteric hepatitis and death.
The presence of antibodies to HAV confers protection against hepatitis A infection. However, the lowest titer needed to confer protection has not been determined.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Pediatric Effectiveness Studies
Protective efficacy with HAVRIX has been demonstrated in a double-blind, randomized controlled study in school children (aged 1 to 16 years) in Thailand who were at high risk of HAV infection. A total of 40,119 children were randomized to be vaccinated with either HAVRIX 360 EL.U. or ENGERIX-B [Hepatitis B Vaccine (Recombinant)] 10 mcg at 0, 1, and 12 months. Of these, 19,037 children received 2 doses of HAVRIX (0 and 1 months) and 19,120 children received 2 doses of control vaccine, ENGERIX-B (0 and 1 months). A total of 38,157 children entered surveillance at Day 138 and were observed for an additional 8 months. Using the protocol-defined endpoint (≥2 days absence from school, ALT level >45 U/mL, and a positive result in the HAVAB-M test), 32 cases of clinical hepatitis A occurred in the control group. In the group receiving HAVRIX, 2 cases were identified. These 2 cases were mild in terms of both biochemical and clinical indices of hepatitis A disease. Thus the calculated efficacy rate for prevention of clinical hepatitis A was 94% (95% Confidence Interval [CI]: 74, 98).
In outbreak investigations occurring in the trial, 26 clinical cases of hepatitis A (of a total of 34 occurring in the trial) occurred. No cases occurred in vaccinees who received HAVRIX.
Using additional virological and serological analyses post hoc, the efficacy of HAVRIX was confirmed. Up to 3 additional cases of mild clinical illness may have occurred in vaccinees. Using available testing, these illnesses could neither be proven nor disproven to have been caused by HAV. By including these as cases, the calculated efficacy rate for prevention of clinical hepatitis A would be 84% (95% CI: 60, 94).
14.2 Immunogenicity in Children and Adolescents
Immune Response to HAVRIX 720 EL.U./0.5 mL at Age 11 to 25 Months (Study HAV 210)
In this prospective, open-label, multicenter study, 1,084 children were administered study vaccine in 1 of 5 groups:
(1) Children aged 11 to 13 months who received HAVRIX on a 0- and 6-month schedule;
(2) Children aged 15 to 18 months who received HAVRIX on a 0- and 6-month schedule;
(3) Children aged 15 to 18 months who received HAVRIX coadministered with INFANRIX and Haemophilus b (Hib) conjugate vaccine (no longer U.S.-licensed) at Month 0 and HAVRIX at Month 6;
(4) Children aged 15 to 18 months who received INFANRIX coadministered with Hib conjugate vaccine at Month 0 and HAVRIX at Months 1 and 7;
(5) Children aged 23 to 25 months who received HAVRIX on a 0- and 6-month schedule.
Among subjects in all groups, 52% were male; 61% of subjects were White, 9% were Black, 3% were Asian, and 27% were other racial/ethnic groups. The anti-hepatitis A antibody vaccine responses and geometric mean antibody titers (GMTs), calculated on responders for Groups 1, 2, and 5 are presented in Table 2. Vaccine response rates were similar among the 3 age-groups that received HAVRIX. One month after the second dose of HAVRIX, the GMT in each of the younger age-groups (aged 11 to 13 months and 15 to 18 months) was shown to be similar to that achieved in the 23- to 25-month age-group.
Table 2. Anti-Hepatitis A Immune Response following 2 Doses of HAVRIX 720 EL.U./0.5 mL Administered 6 Months Apart in Children Given the First Dose of HAVRIX at Age 11 to 13 Months, 15 to 18 Months, or 23 to 25 Months Vaccine response = Seroconversion (anti-HAV ≥15 mIU/mL [lower limit of antibody measurement by assay]) in children initially seronegative or at least the maintenance of the pre-vaccination anti-HAV concentration in initially seropositive children. CI = Confidence Interval; GMT = Geometric mean antibody titer. a Calculated on vaccine responders 1 month post-dose 2. GMTs in children aged 11 to 13 months and 15 to 18 months were non-inferior (similar) to the GMT in children aged 23 to 25 months (i.e., the lower limit of the 2-sided 95% CI on the GMT ratio for Group 1/Group 5 and for Group 2/Group 5 were both ≥0.5). Age Group
n
Vaccine Response
GMT
(mIU/mL)
%
95% CI
11-13 months (Group 1)
218
99
97, 100
1,461a
15-18 months (Group 2)
200
100
98, 100
1,635a
23-25 months (Group 5)
211
100
98, 100
1,911
In 3 additional clinical studies (HAV 232, HAV 220, and HAV 231), children received either 2 doses of HAVRIX alone or the first dose of HAVRIX concomitantly administered with other routinely recommended U.S.-licensed vaccines followed by a second dose of HAVRIX. After the second dose of HAVRIX, there was no evidence for interference with the anti-HAV response in the children who received concomitantly administered vaccines compared with those who received HAVRIX alone. [See Adverse Reactions (6.1), Clinical Studies (14.5).]
Immune Response to HAVRIX 360 EL.U. among Individuals Aged 2 to 18 Years
In 6 clinical studies, 762 subjects aged 2 to 18 years received 2 doses of HAVRIX (360 EL.U.) given 1 month apart (GMT ranged from 197 to 660 mIU/mL). Ninety-nine percent of subjects seroconverted following 2 doses. When a third dose of HAVRIX 360 EL.U. was administered 6 months following the initial dose, all subjects were seropositive (anti-HAV ≥20 mIU/mL) 1 month following the third dose, with GMTs rising to a range of 3,388 to 4,643 mIU/mL. In 1 study in which children were followed for an additional 6 months, all subjects remained seropositive.
Immune Response to HAVRIX 720 EL.U./0.5 mL among Individuals Aged 2 to 19 Years
In 4 clinical studies, 314 children and adolescents ranging from age 2 to 19 years were immunized with 2 doses of HAVRIX 720 EL.U./0.5 mL given 6 months apart. One month after the first dose, seroconversion (anti-HAV ≥20 mIU/mL [lower limit of antibody measurement by assay]) ranged from 96.8% to 100%, with GMTs of 194 mIU/mL to 305 mIU/mL. In studies in which sera were obtained 2 weeks following the initial dose, seroconversion ranged from 91.6% to 96.1%. One month following the booster dose at Month 6, all subjects were seropositive, with GMTs ranging from 2,495 mIU/mL to 3,644 mIU/mL.
In an additional study in which the booster dose was delayed until 1 year following the initial dose, 95.2% of the subjects were seropositive just prior to administration of the booster dose. One month later, all subjects were seropositive, with a GMT of 2,657 mIU/mL.
14.3 Immunogenicity in Adults
More than 400 healthy adults aged 18 to 50 years in 3 clinical studies were given a single 1440 EL.U. dose of HAVRIX. All subjects were seronegative for hepatitis A antibodies at baseline. Specific humoral antibodies against HAV were elicited in more than 96% of subjects when measured 1 month after vaccination. By Day 15, 80% to 98% of vaccinees had already seroconverted (anti-HAV ≥20 mIU/mL [lower limit of antibody measurement by assay]). GMTs of seroconverters ranged from 264 to 339 mIU/mL at Day 15 and increased to a range of 335 to 637 mIU/mL by 1 month following vaccination.
The GMTs obtained following a single dose of HAVRIX are at least several times higher than that expected following receipt of immune globulin.
In a clinical study using 2.5 to 5 times the standard dose of immune globulin (standard dose = 0.02 to 0.06 mL/kg), the GMT in recipients was 146 mIU/mL at 5 days post-administration, 77 mIU/mL at Month 1, and 63 mIU/mL at Month 2.
In 2 clinical trials in which a booster dose of 1440 EL.U. was given 6 months following the initial dose, 100% of vaccinees (n = 269) were seropositive 1 month after the booster dose, with GMTs ranging from 3,318 mIU/mL to 5,925 mIU/mL. The titers obtained from this additional dose approximate those observed several years after natural infection.
In a subset of vaccinees (n = 89), a single dose of HAVRIX 1440 EL.U. elicited specific anti-HAV neutralizing antibodies in more than 94% of vaccinees when measured 1 month after vaccination. These neutralizing antibodies persisted until Month 6. One hundred percent of vaccinees had neutralizing antibodies when measured 1 month after a booster dose given at Month 6.
Immunogenicity of HAVRIX was studied in subjects with chronic liver disease of various etiologies. One hundred eighty-nine healthy adults and 220 adults with either chronic hepatitis B (n = 46), chronic hepatitis C (n = 104), or moderate chronic liver disease of other etiology (n = 70) were vaccinated with HAVRIX 1440 EL.U. on a 0- and 6-month schedule. The last group consisted of alcoholic cirrhosis (n = 17), autoimmune hepatitis (n = 10), chronic hepatitis/cryptogenic cirrhosis (n = 9), hemochromatosis (n = 2), primary biliary cirrhosis (n = 15), primary sclerosing cholangitis (n = 4), and unspecified (n = 13). At each time point, GMTs were lower for subjects with chronic liver disease than for healthy subjects. At Month 7, the GMTs ranged from 478 mIU/mL (chronic hepatitis C) to 1,245 mIU/mL (healthy). One month after the first dose, seroconversion rates in adults with chronic liver disease were lower than in healthy adults. However, 1 month after the booster dose at Month 6, seroconversion rates were similar in all groups; rates ranged from 94.7% to 98.1%. The relevance of these data to the duration of protection afforded by HAVRIX is unknown.
In subjects with chronic liver disease, local injection site reactions with HAVRIX were similar among all 4 groups, and no serious adverse reactions attributed to the vaccine were reported in subjects with chronic liver disease.
14.4 Duration of Immunity
The duration of immunity following a complete schedule of immunization with HAVRIX has not been established.
14.5 Immune Response to Concomitantly Administered Vaccines
In 3 clinical studies HAVRIX was administered concomitantly with other routinely recommended U.S.-licensed vaccines: Study HAV 232: Diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed (INFANRIX, DTaP) and Haemophilus b (Hib) conjugate vaccine (tetanus toxoid conjugate) (manufactured by Sanofi Pasteur SA); Study HAV 220: Pneumococcal 7-valent conjugate vaccine (PCV-7) (manufactured by Pfizer), and Study HAV 231: MMR and varicella vaccines. [See Adverse Reactions (6.1).]
Concomitant Administration with DTaP and Hib Conjugate Vaccine (Study HAV 232)
In this U.S. multicenter study, 468 subjects, children aged 15 months were randomized to receive: Group 1) HAVRIX coadministered with INFANRIX and Hib conjugate vaccine (n = 127); Group 2) INFANRIX and Hib conjugate vaccine alone followed by a first dose of HAVRIX 1 month later (n = 132); or Group 3) HAVRIX alone (n = 135). All subjects received a second dose of HAVRIX alone 6 to 9 months following the first dose. Among subjects in all groups combined, 53% were male; 64% of subjects were White, 12% were Black, 6% were Hispanic, and 18% were other racial/ethnic groups.
There was no evidence for reduced antibody response to diphtheria and tetanus toxoids (percentage of subjects with antibody levels ≥0.1 mIU/mL to each antigen), pertussis antigens (percentage of subjects with seroresponse, antibody concentrations ≥5 EL.U./mL in seronegative subjects or post-vaccination antibody concentration ≥2 times the pre-vaccination antibody concentration in seropositive subjects, and GMTs), or Hib (percentage of subjects with antibody levels ≥1 mcg/mL to polyribosyl-ribitol phosphate, PRP) when HAVRIX was administered concomitantly with INFANRIX and Hib conjugate vaccine (Group 1) relative to INFANRIX and Hib conjugate vaccine administered together (Group 2).
Concomitant Administration with Pneumococcal 7-Valent Conjugate Vaccine (Study HAV 220)
In this U.S. multicenter study, 433 children aged 15 months were randomized to receive: Group 1) HAVRIX coadministered with PCV-7 vaccine (n = 137); Group 2) HAVRIX administered alone (n = 147); or Group 3) PCV-7 vaccine administered alone (n = 149) followed by a first dose of HAVRIX 1 month later. All subjects received a second dose of HAVRIX 6 to 9 months after the first dose. Among subjects in all groups combined, 53% were female; 61% of subjects were White, 16% were Hispanic, 15% were Black, and 8% were other racial/ethnic groups.
There was no evidence for reduced antibody response to PCV-7 (GMC to each serotype) when HAVRIX was administered concomitantly with PCV-7 vaccine (Group 1) relative to PCV-7 administered alone (Group 3).
Concomitant Administration with MMR and Varicella Vaccines (Study HAV 231)
In a U.S. multicenter study, there was no evidence for interference in the immune response to MMR and varicella vaccines (the percentage of subjects with pre-specified seroconversion/seroresponse levels) administered to subjects aged 15 months concomitantly with HAVRIX relative to the response when MMR and varicella vaccines are administered without HAVRIX. [See Adverse Reactions (6.1).]
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
HAVRIX is available in single-dose prefilled disposable TIP-LOK syringes (Luer Lock syringes) packaged without needles (Preservative-Free Formulation). TIP-LOK syringes are to be used with Luer Lock compatible needles. The tip cap and rubber plunger stopper of the prefilled syringe are not made with natural rubber latex.
720 EL.U./0.5 mL
NDC 58160-825-43 Prefilled Syringe in Package of 10: NDC 58160-825-52
1440 EL.U./mL
NDC 58160-826-43 Prefilled Syringe in Package of 10: NDC 58160-826-52
Store refrigerated between 2° and 8°C (36° and 46°F). Do not freeze. Discard if the vaccine has been frozen. Do not dilute to administer.
-
17 PATIENT COUNSELING INFORMATION
- •
- Inform vaccine recipients and parents or guardians of the potential benefits and risks of immunization with HAVRIX.
- •
- Emphasize, when educating vaccine recipients and parents or guardians regarding potential side effects, that HAVRIX contains non-infectious killed viruses and cannot cause hepatitis A infection.
- •
- Instruct vaccine recipients and parents or guardians to report any adverse events to their healthcare provider.
- •
- Give vaccine recipients and parents or guardians the Vaccine Information Statements, which are required by the National Childhood Vaccine Injury Act of 1986 to be given prior to immunization. These materials are available free of charge at the Centers for Disease Control and Prevention (CDC) website (www.cdc.gov/vaccines).
Trademarks are owned by or licensed to the GSK group of companies.
Manufactured by GlaxoSmithKline Biologicals
Rixensart, Belgium, U.S. License No. 1617
Distributed by GlaxoSmithKline
Durham, NC 27701
©2023 GSK group of companies or its licensor.
HVX:48PI
-
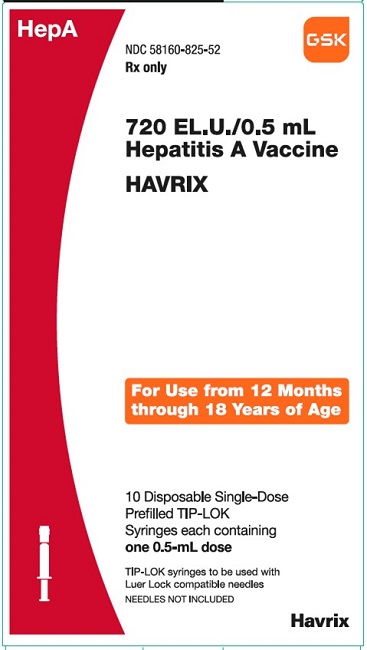
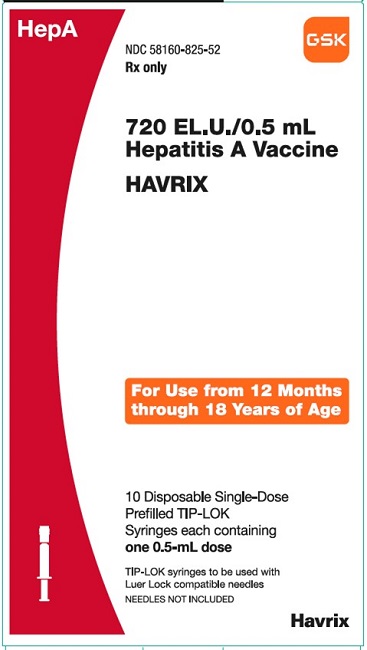
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 58160-825-52
HAVRIX
720 EL.U./0.5 mL
Hepatitis A Vaccine
HepA
Rx only
For Use from 12 Months through 18 Years of Age
10 Disposable Single-Dose Prefilled TIP-LOK Syringes each containing one 0.5-mL dose
TIP-LOK syringes to be used with Luer Lock compatible needles
NEEDLES NOT INCLUDED
GSK
Havrix
Made in Belgium
©2023 GSK group of companies or its licensor.
Rev.10/23
515876
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 58160-826-52
HAVRIX
1440 EL.U./mL
Hepatitis A Vaccine
HepA
Rx only
For 19 Years of Age and Older
10 Disposable Single-Dose Prefilled TIP-LOK Syringes each containing one 1-mL dose
TIP-LOK syringes to be used with Luer Lock compatible needles
NEEDLES NOT INCLUDED
GSK
Havrix
Made in Belgium
©2023 the GSK group of companies or its licensor.
Rev. 10/23
515894
-
INGREDIENTS AND APPEARANCE
HAVRIX
hepatitis a vaccine injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:58160-825 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS A VIRUS STRAIN HM175 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 5BFC8LZ6LQ) (HEPATITIS A VIRUS STRAIN HM175 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:5BFC8LZ6LQ) HEPATITIS A VIRUS STRAIN HM175 ANTIGEN (FORMALDEHYDE INACTIVATED) 720 [iU] in 0.5 mL Inactive Ingredients Ingredient Name Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYSORBATE 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-825-52 10 in 1 CARTON 1 NDC:58160-825-43 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103475 02/16/2007 HAVRIX
hepatitis a vaccine injection, suspensionProduct Information Product Type VACCINE Item Code (Source) NDC:58160-826 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HEPATITIS A VIRUS STRAIN HM175 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 5BFC8LZ6LQ) (HEPATITIS A VIRUS STRAIN HM175 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:5BFC8LZ6LQ) HEPATITIS A VIRUS STRAIN HM175 ANTIGEN (FORMALDEHYDE INACTIVATED) 1440 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYSORBATE 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58160-826-52 10 in 1 CARTON 1 NDC:58160-826-43 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103475 04/13/2007 Labeler - GlaxoSmithKline Biologicals SA (372748392)