Label: ACNE MOISTURIZER- salycilic acid cream
- NDC Code(s): 78518-012-01
- Packager: MONAT GLOBAL CORP
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- USES
-
Warnings
Warnings
For external use only
When using this product
When using this product
- skin irritation and dryness are more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne treatment at a time.
- Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
-
DIRECTIONS
Directions
- Clean skin thoroughly before applying this product
- Apply a dime-sized amount onto fingers. Gently use fingertips to apply to clean and dry face using circular motions
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
Inactive Ingredients
Inactive Ingredients: Water, Dimethyl Isosorbide, Glycerin, Sodium Hydroxide, Cetearyl Olivate, Caprylic/Capric Triglyceride, Lactococcus Ferment Lysate, Niacinamide, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Sorbitan Isostearate, Polysorbate 60, Polyacrylate Crosspolymer-6, Sorbitan Olivate, Jojoba Esters, Helianthus Annuus (Sunflower) Seed Wax, Acacia Decurrens Flower Wax, Polyglycerin-3, Tocopherol, Coco-Caprylate/Caprate, Triheptanoin, C9-12 Alkane, Dilinoleic Acid/Butanediol Copolymer, Castor Oil/IPDI Copolymer, Physalis Angulata Extract, Lactic Acid, Sodium Hyaluronate, 1,2-Hexanediol, Polylysine, Camellia Sinensis Leaf Extract, Potassium Sorbate, Sodium Benzoate, Bisabolol, Pentylene Glycol, Propanediol, Caprylyl Glycol, Ethylhexylglycerin, Sodium Chloride, t-Butyl Alcohol, Chlorphenesin, Phenylpropanol, Levulinic Acid, Sodium Levulinate
- Questions or comments
- Primary Package
- Secondary Package
-
INGREDIENTS AND APPEARANCE
ACNE MOISTURIZER
salycilic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78518-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mg in 100 mg Inactive Ingredients Ingredient Name Strength TOCOPHEROL (UNII: R0ZB2556P8) DILINOLEIC ACID/BUTANEDIOL COPOLYMER (UNII: 1F2S8T535O) SODIUM LEVULINATE (UNII: VK44E1MQU8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) LEVOMENOL (UNII: 24WE03BX2T) SODIUM CHLORIDE (UNII: 451W47IQ8X) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) LEVULINIC ACID (UNII: RYX5QG61EI) POLY-L-LYSINE (30000-70000 MW) (UNII: 0A1V8JTU2M) NIACINAMIDE (UNII: 25X51I8RD4) PROPANEDIOL (UNII: 5965N8W85T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) PHENYLPROPANOL (UNII: 0F897O3O4M) GREEN TEA LEAF (UNII: W2ZU1RY8B0) PHYSALIS ANGULATA (UNII: W4TKW9D5GG) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CETEARYL OLIVATE (UNII: 58B69Q84JO) LACTIC ACID (UNII: 33X04XA5AT) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) TRIHEPTANOIN (UNII: 2P6O7CFW5K) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) SORBITAN OLIVATE (UNII: MDL271E3GR) HYDROLYZED JOJOBA ESTERS (POTASSIUM SALTS) (UNII: CH428W5O62) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PENTYLENE GLYCOL (UNII: 50C1307PZG) SODIUM HYDROXIDE (UNII: 55X04QC32I) CAPRYLIC/CAPRIC/LINOLEIC TRIGLYCERIDE (UNII: U73D397055) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) LACTOCOCCUS LACTIS (UNII: F1A0PSN10V) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) POLYSORBATE 60 (UNII: CAL22UVI4M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78518-012-01 1 in 1 CARTON 04/04/2023 1 50 mg in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 04/04/2023 Labeler - MONAT GLOBAL CORP (027036949) Establishment Name Address ID/FEI Business Operations COSMAX USA, INC 010990210 manufacture(78518-012)

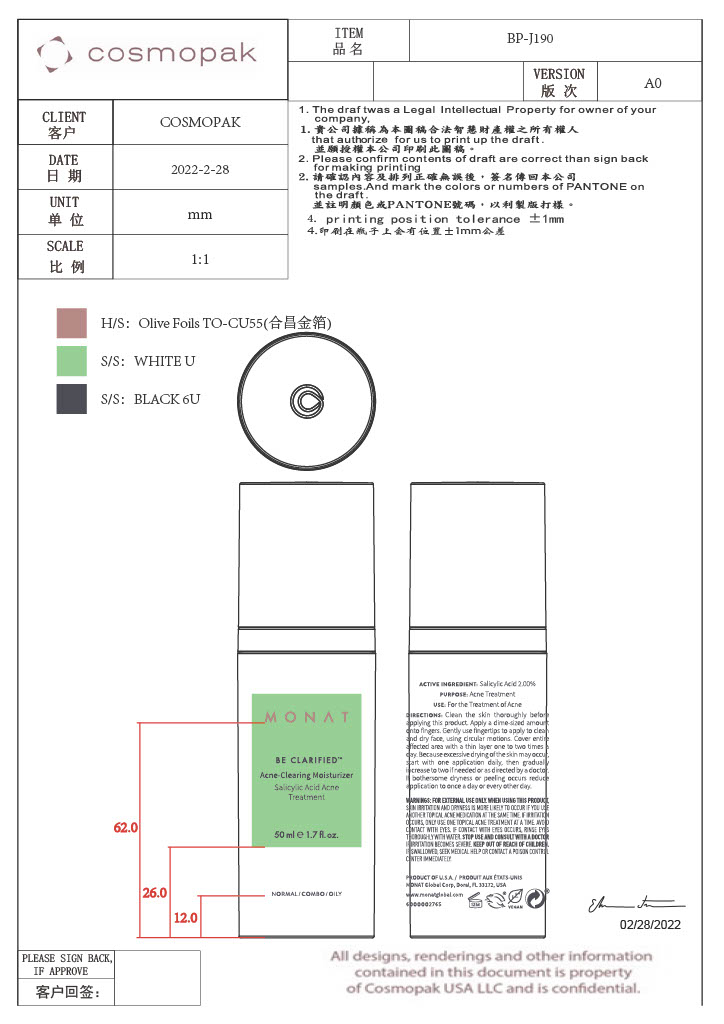

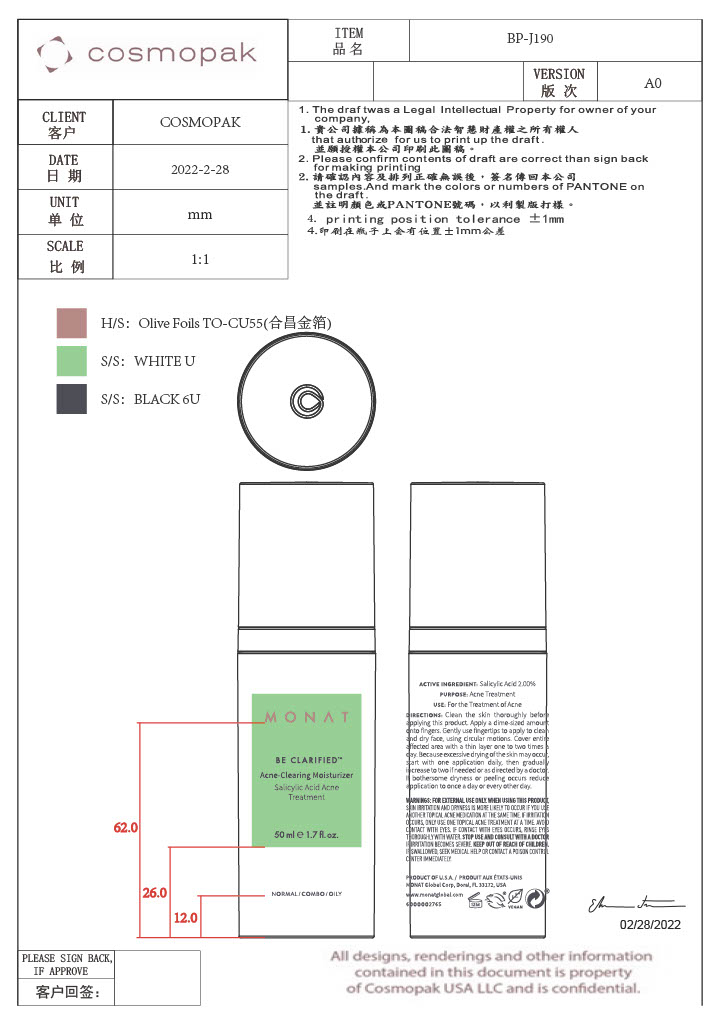 Primary Package
Primary Package
 Secondary Package
Secondary Package