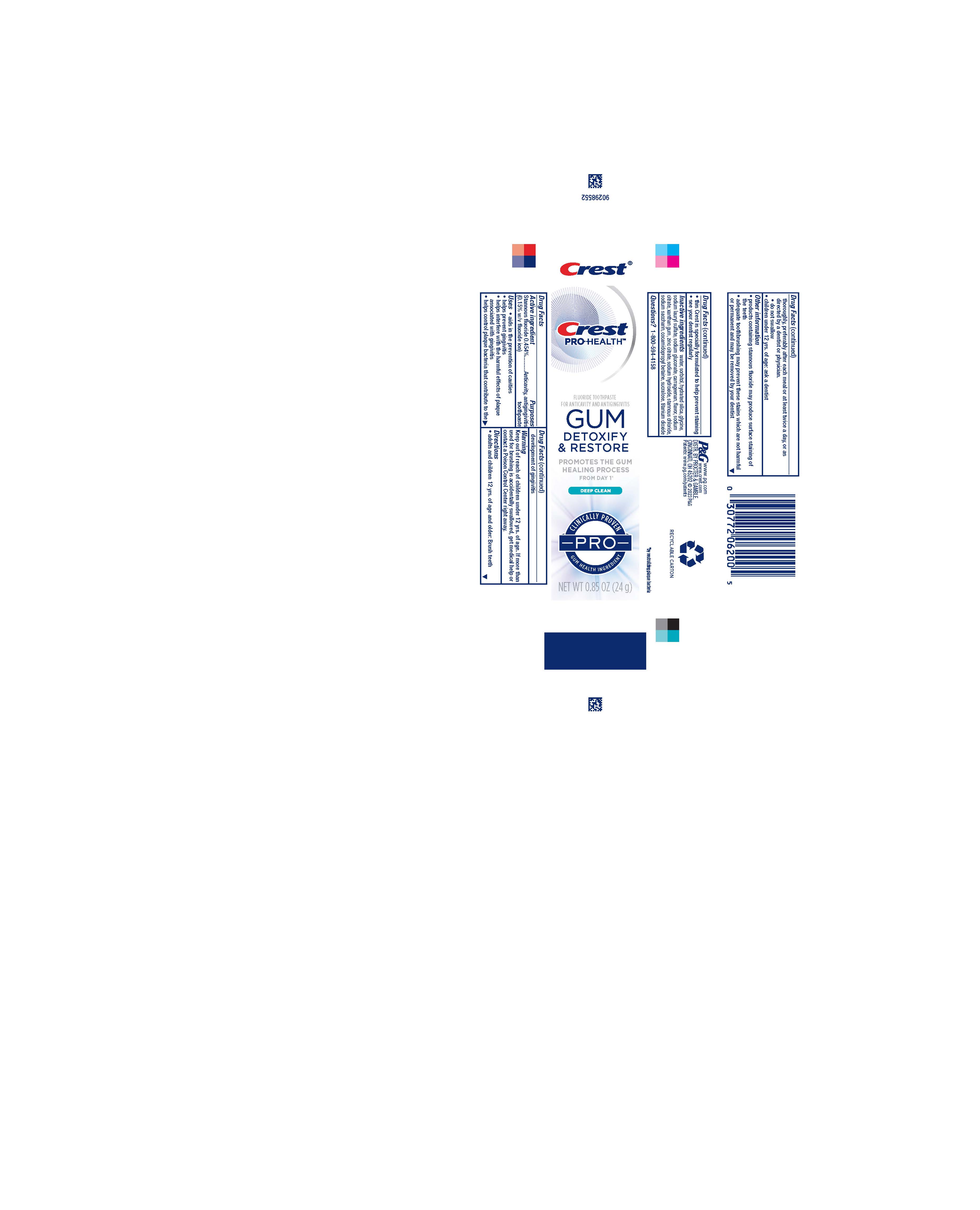
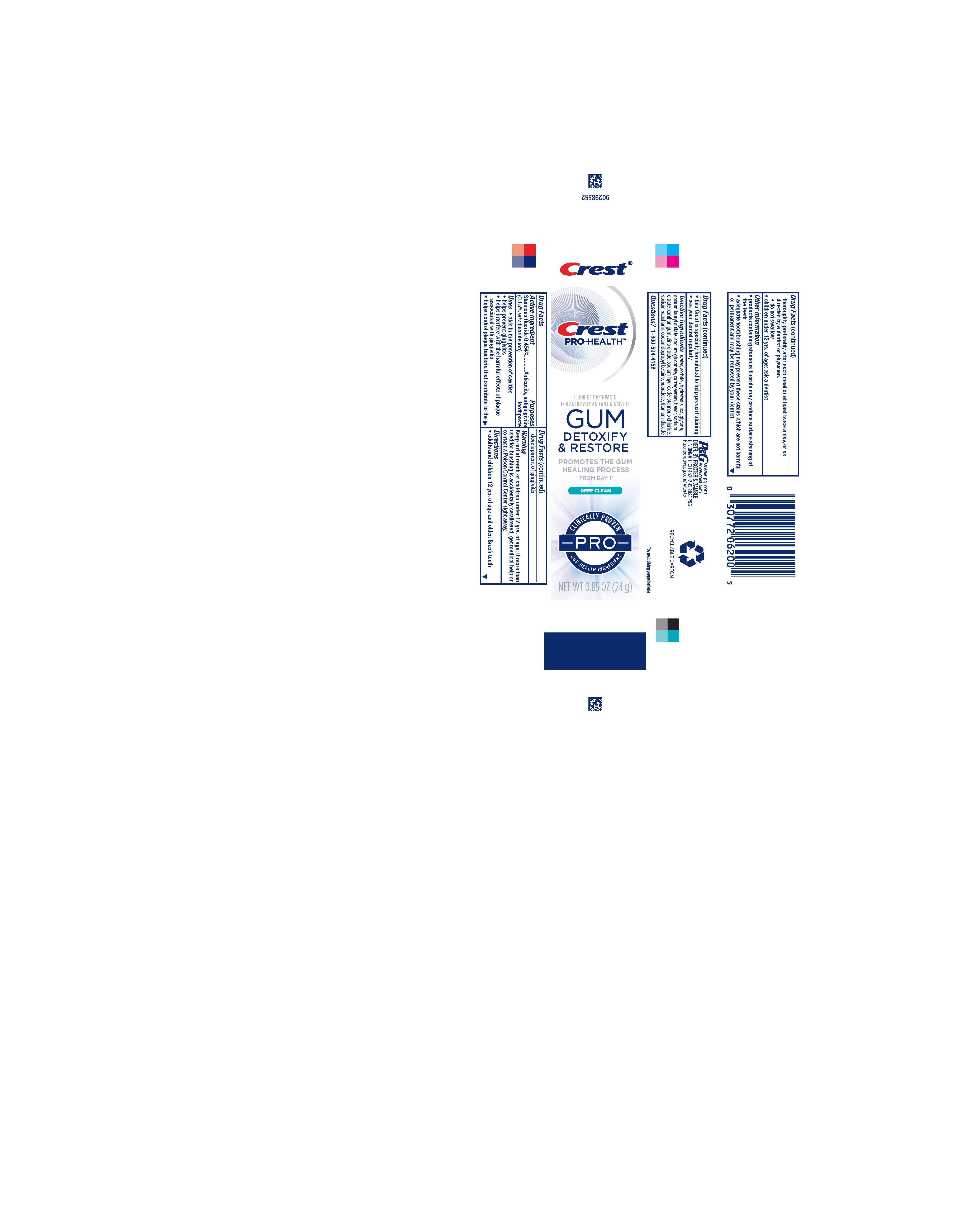
Label: CREST PRO HEALTH GUM DETOXIFY AND RESTORE PRO DEEP CLEAN- stannous fluoride paste, dentifrice
- NDC Code(s): 69423-763-25, 69423-763-35, 69423-763-72, 69423-763-85
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purposes
- Uses
- Warning
- Directions
- KEEP OUT OF REACH OF CHILDREN
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Principal Display Panel - 24 g tube in carton
-
INGREDIENTS AND APPEARANCE
CREST PRO HEALTH GUM DETOXIFY AND RESTORE PRO DEEP CLEAN
stannous fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69423-763 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength SUCRALOSE (UNII: 96K6UQ3ZD4) STANNOUS CHLORIDE (UNII: 1BQV3749L5) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) CARRAGEENAN (UNII: 5C69YCD2YJ) SODIUM GLUCONATE (UNII: R6Q3791S76) XANTHAN GUM (UNII: TTV12P4NEE) ZINC CITRATE (UNII: K72I3DEX9B) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SORBITOL (UNII: 506T60A25R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SACCHARIN SODIUM (UNII: SB8ZUX40TY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM CITRATE (UNII: 1Q73Q2JULR) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) Product Characteristics Color white Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69423-763-85 1 in 1 CARTON 02/15/2023 1 24 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69423-763-25 1 in 1 CARTON 02/15/2023 2 70 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:69423-763-35 1 in 1 CARTON 02/15/2023 3 99 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC:69423-763-72 3 in 1 CELLO PACK 02/15/2023 4 1 in 1 CARTON 4 99 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 02/15/2023 Labeler - The Procter & Gamble Manufacturing Company (004238200)