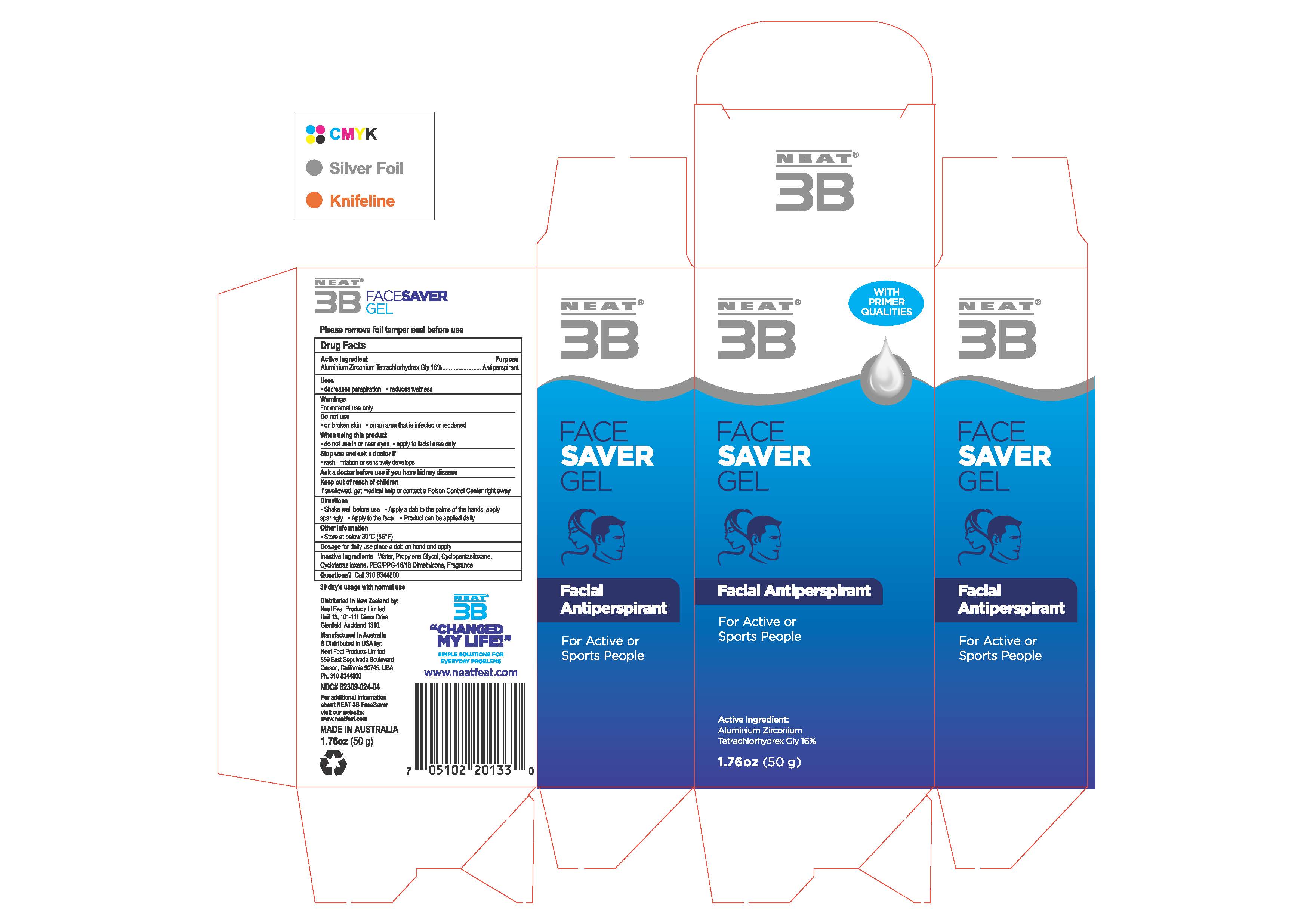
Label: FACE SAVER GEL- antiperspirant gel
- NDC Code(s): 50017-024-01
- Packager: Neat Feat Products Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
NDC# 82309-024-04
Do not use:
-on broken skin - on an area that is infected or reddened
When using this product
• do not use in or near eyes •apply to facial area only
Stop use and ask a doctor if
• rash, irritation or sensitivity develops
Ask a doctor before use if you have kidney disease
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away -
INGREDIENTS AND APPEARANCE
FACE SAVER GEL
antiperspirant gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50017-024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY (UNII: T27D6T99LH) (ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY - UNII:T27D6T99LH) ALUMINUM ZIRCONIUM TRICHLOROHYDREX GLY 8.75 g in 50 g Inactive Ingredients Ingredient Name Strength PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 4 (UNII: CZ227117JE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50017-024-01 50 in 1 CARTON 02/01/2022 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 12/01/2021 Labeler - Neat Feat Products Limited (590412409)