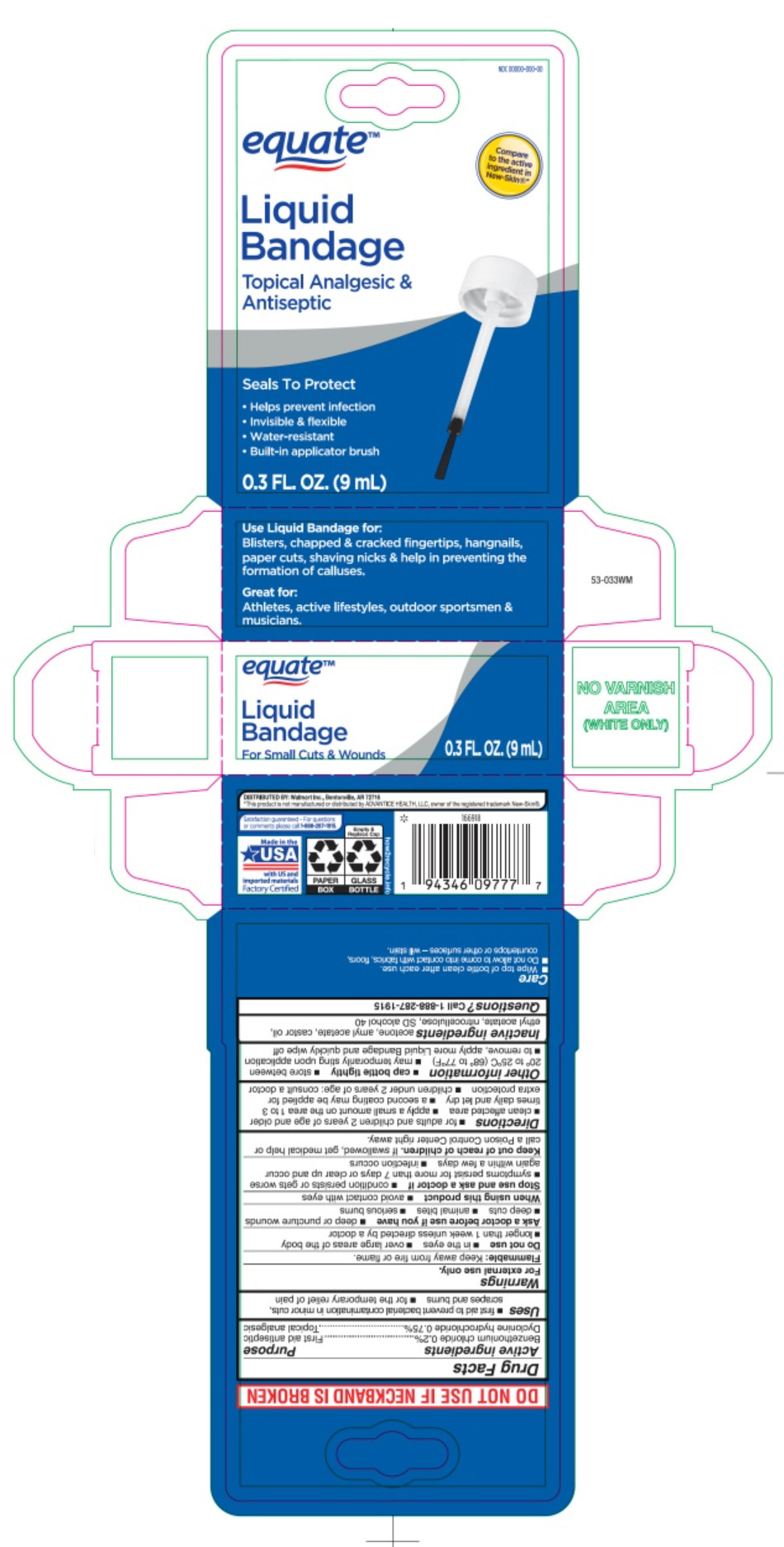
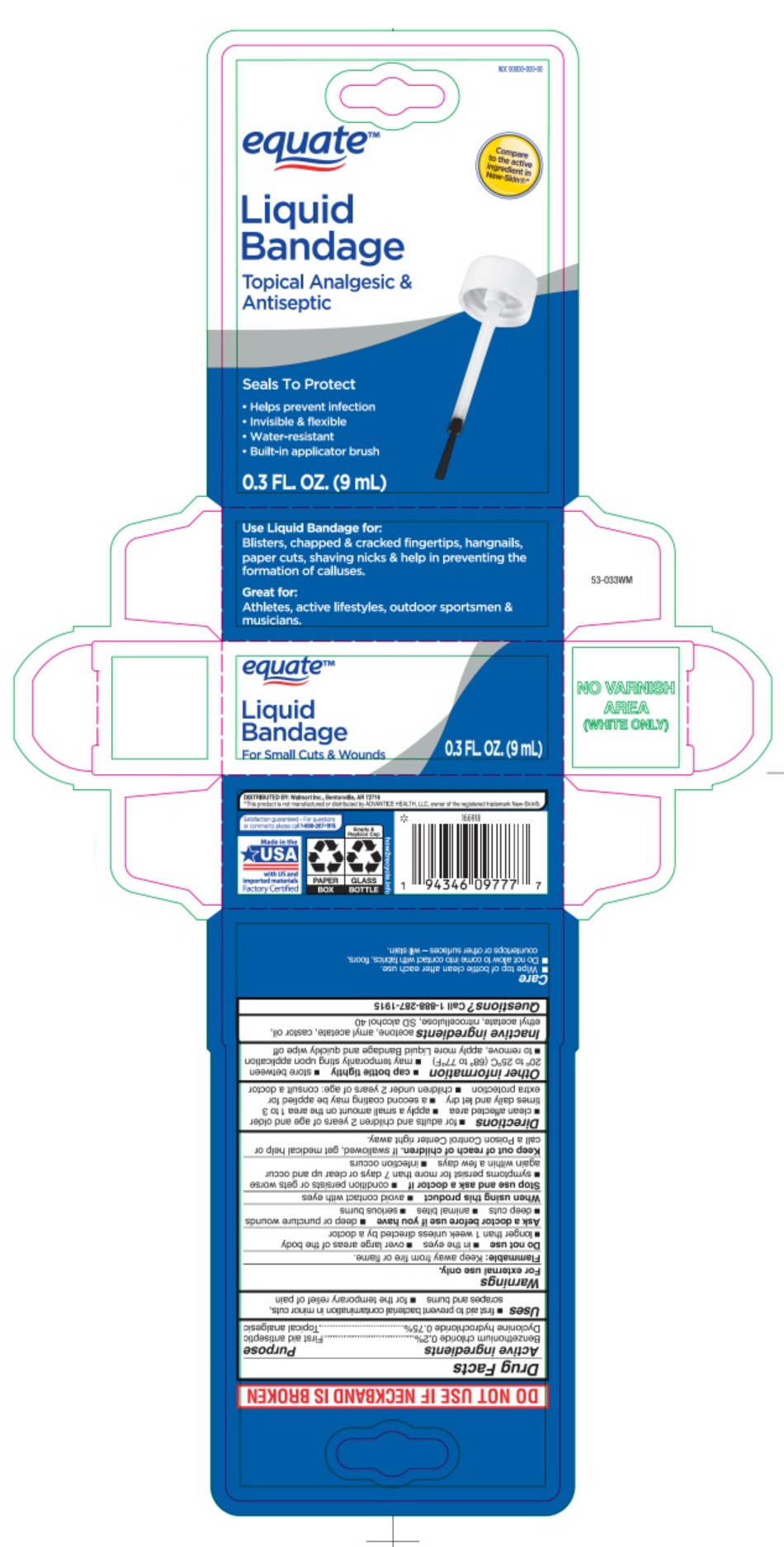
Label: LIQUID BANDAGE- benzethonium chloride and dyclonine hydrochloride liquid
- NDC Code(s): 79903-485-30
- Packager: Wal-Mart Stores, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
LIQUID BANDAGE
benzethonium chloride and dyclonine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-485 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.06 mg in 29.6 mL DYCLONINE HYDROCHLORIDE (UNII: ZEC193879Q) (DYCLONINE - UNII:078A24Q30O) DYCLONINE HYDROCHLORIDE 0.23 mg in 29.6 mL Inactive Ingredients Ingredient Name Strength ACETONE (UNII: 1364PS73AF) AMYL ACETATE (UNII: 92Q24NH7AS) CASTOR OIL (UNII: D5340Y2I9G) ETHYL ACETATE (UNII: 76845O8NMZ) PYROXYLIN (UNII: KYR8BR2X6O) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-485-30 29.6 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 01/31/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/12/2012 Labeler - Wal-Mart Stores, Inc. (051957769)